This page is based on this
Wikipedia article Text is available under the
CC BY-SA 4.0 license; additional terms may apply.
Images, videos and audio are available under their respective licenses.
Chlorine is a chemical element with symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between them. Chlorine is a yellow-green gas at room temperature. It is an extremely reactive element and a strong oxidising agent: among the elements, it has the highest electron affinity and the third-highest electronegativity, behind only oxygen and fluorine.

Perchloric acid is a mineral acid with the formula HClO4. Usually found as an aqueous solution, this colorless compound is a stronger acid than sulfuric acid and nitric acid. It is a powerful oxidizer when hot, but aqueous solutions up to approximately 70% by weight at room temperature are generally safe, only showing strong acid features and no oxidizing properties. Perchloric acid is useful for preparing perchlorate salts, especially ammonium perchlorate, an important rocket fuel component. Perchloric acid is dangerously corrosive and readily forms potentially explosive mixtures.

Aqua regia is a mixture of nitric acid and hydrochloric acid, optimally in a molar ratio of 1:3. Aqua regia is a yellow-orange fuming liquid, so named by alchemists because it can dissolve the noble metals gold and platinum, though not all metals.
The chlorite ion, or chlorine dioxide anion, is ClO−
2. A chlorite (compound) is a compound that contains this group,
with chlorine in oxidation state +3. Chlorites are also known as salts of chlorous acid.
The compound hydrogen chloride has the chemical formula HCl and as such is a hydrogen halide. At room temperature, it is a colourless gas, which forms white fumes of hydrochloric acid upon contact with atmospheric water vapor. Hydrogen chloride gas and hydrochloric acid are important in technology and industry. Hydrochloric acid, the aqueous solution of hydrogen chloride, is also commonly given the formula HCl.

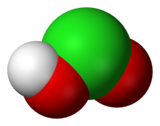
Chloric acid, HClO3, is an oxoacid of chlorine, and the formal precursor of chlorate salts. It is a strong acid (pKa ≈ −1) and oxidizing agent.
A mineral acid is an acid derived from one or more inorganic compounds. All mineral acids form hydrogen ions and the conjugate base when dissolved in water.
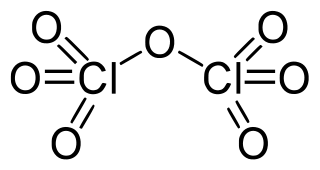
Dichlorine heptoxide is the chemical compound with the formula Cl2O7. This chlorine oxide is the anhydride of perchloric acid. It is produced by the careful distillation of perchloric acid in the presence of the dehydrating agent phosphorus pentoxide:

Sodium Chlorite (NaClO2) is a chemical compound used in the manufacturing of paper and as a disinfectant.

Iodic acid, HIO3, can be obtained as a white or off-white solid. It dissolves in water very well, but it also exists in the pure state, as opposed to chloric acid or bromic acid. Iodic acid contains iodine in the oxidation state +5 and is one of the most stable oxo-acids of the halogens in its pure state. When iodic acid is carefully heated, it dehydrates to iodine pentoxide. On subsequent heating, the iodine pentoxide further decomposes, giving a mix of iodine, oxygen and lower oxides of iodine.
A strong electrolyte is a solution/solute that completely, or almost completely, ionizes or dissociates in a solution. These ions are good conductors of electric current in the solution.
Calcium hypochlorite is an inorganic compound with formula Ca(ClO)2. It is the main active ingredient of commercial products called bleaching powder, chlorine powder, or chlorinated lime, used for water treatment and as a bleaching agents. This compound is relatively stable and has greater available chlorine than sodium hypochlorite (liquid bleach). It is a white solid, although commercial samples appear yellow. It strongly smells of chlorine, owing to its slow decomposition in moist air. It is not highly soluble in hard water, and is more preferably used in soft to medium-hard water. It has two forms: dry (anhydrous); and hydrated (hydrous).
An oxyacid, or oxoacid, is an acid that contains oxygen. Specifically, it is a compound that contains hydrogen, oxygen, and at least one other element, with at least one hydrogen
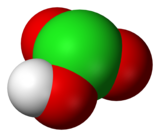
Calcium chlorate is the calcium salt of chloric acid, with the chemical formula Ca(ClO3)2. Like other chlorates, it is a strong oxidizer.

Dichlorine hexoxide is the chemical compound with the molecular formula Cl
2O
6, which is correct for its gaseous state. However, in liquid or solid form, this chlorine oxide ionizes into the dark red ionic compound chloryl perchlorate [ClO
2]+
[ClO
4]−
, which may be thought of as the mixed anhydride of chloric and perchloric acids.
An oxidizing acid is a Brønsted acid that is a strong oxidizing agent. All Brønsted acids can act as oxidizing agents, because the acidic proton can be reduced to hydrogen gas. Some acids contain other structures that act as stronger oxidizing agents than hydrogen ion. Generally, they contain oxygen in the anionic structure. These include nitric acid, perchloric acid, chloric acid, chromic acid, and concentrated sulfuric acid, among others.
The Pinnick oxidation is an organic reaction by which aldehydes can be oxidized into their corresponding carboxylic acids using sodium chlorite (NaClO2) under mild acidic conditions. It was originally developed by Lindgren and Nilsson. The typical reaction conditions used today were developed by G. A. Kraus. H.W. Pinnick later demonstrated that these conditions could be applied to oxidize α,β-unsaturated aldehydes. There exist many different reactions to oxidize aldehydes, but only a few are amenable to a broad range of functional groups. The Pinnick oxidation has proven to be both tolerant of sensitive functionalities and capable of reacting with sterically hindered groups. This reaction is especially useful for oxidizing α,β-unsaturated aldehydes, and another one of its advantages is its relatively low cost.