An aromatic hydrocarbon or arene is a hydrocarbon with sigma bonds and delocalized pi electrons between carbon atoms forming a circle. In contrast, aliphatic hydrocarbons lack this delocalization. The term "aromatic" was assigned before the physical mechanism determining aromaticity was discovered; the term was coined as such simply because many of the compounds have a sweet or pleasant odour. The configuration of six carbon atoms in aromatic compounds is known as a benzene ring, after the simplest possible such hydrocarbon, benzene. Aromatic hydrocarbons can be monocyclic (MAH) or polycyclic (PAH).
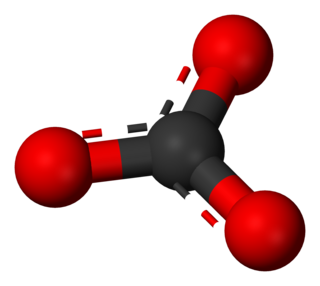
In chemistry, a carbonate is a salt of carbonic acid (H2CO3), characterized by the presence of the carbonate ion, a polyatomic ion with the formula of CO2−
3. The name may also refer to a carbonate ester, an organic compound containing the carbonate group C(=O)(O–)2.
A chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, such as parentheses, dashes, brackets, commas and plus (+) and minus (−) signs. These are limited to a single typographic line of symbols, which may include subscripts and superscripts. A chemical formula is not a chemical name, and it contains no words. Although a chemical formula may imply certain simple chemical structures, it is not the same as a full chemical structural formula. Chemical formulas can fully specify the structure of only the simplest of molecules and chemical substances, and are generally more limited in power than are chemical names and structural formulas.

In chemistry, an ester is a chemical compound derived from an acid in which at least one –OH (hydroxyl) group is replaced by an –O–alkyl (alkoxy) group. Usually, esters are derived from a carboxylic acid and an alcohol. Glycerides, which are fatty acid esters of glycerol, are important esters in biology, being one of the main classes of lipids, and making up the bulk of animal fats and vegetable oils. Esters with low molecular weight are commonly used as fragrances and found in essential oils and pheromones. Phosphoesters form the backbone of DNA molecules. Nitrate esters, such as nitroglycerin, are known for their explosive properties, while polyesters are important plastics, with monomers linked by ester moieties. Esters usually have a sweet smell and are considered high-quality solvents for a broad array of plastics, plasticizers, resins, and lacquers. They are also one of the largest classes of synthetic lubricants on the commercial market.

In organic chemistry, functional groups are specific substituents or moieties within molecules that are responsible for the characteristic chemical reactions of those molecules. The same functional group will undergo the same or similar chemical reaction(s) regardless of the size of the molecule it is a part of. This allows for systematic prediction of chemical reactions and behavior of chemical compounds and design of chemical syntheses. Furthermore, the reactivity of a functional group can be modified by other functional groups nearby. In organic synthesis, functional group interconversion is one of the basic types of transformations.
In chemistry, the law of multiple proportions is one of the basic laws of stoichiometry used to establish the atomic theory, alongside the law of conservation of mass (matter) and the law of definite proportions. It is sometimes called Dalton's Law after its discoverer, the British chemist John Dalton, who published it in the first part of the first volume of his "New System of Chemical Philosophy" (1808). Here is the statement of the law:
Monosaccharides, also called simple sugars, are the simplest form of sugar and the most basic units of carbohydrates. They cannot be further hydrolyzed to simpler chemical compounds. The general formula is C
nH
2nO
n. They are usually colorless, water-soluble, and crystalline solids. Some monosaccharides have a sweet taste.

Redox is a chemical reaction in which the oxidation states of atoms are changed. Any such reaction involves both a reduction process and a complementary oxidation process, two key concepts involved with electron transfer processes. Redox reactions include all chemical reactions in which atoms have their oxidation state changed; in general, redox reactions involve the transfer of electrons between chemical species. The chemical species from which the electron is stripped is said to have been oxidized, while the chemical species to which the electron is added is said to have been reduced. It can be explained in simple terms:
Lewis structures, also known as Lewis dot diagrams, Lewis dot formulas, Lewis dot structures, electron dot structures, or Lewis electron dot structures (LEDS), are diagrams that show the bonding between atoms of a molecule and the lone pairs of electrons that may exist in the molecule. A Lewis structure can be drawn for any covalently bonded molecule, as well as coordination compounds. The Lewis structure was named after Gilbert N. Lewis, who introduced it in his 1916 article The Atom and the Molecule. Lewis structures extend the concept of the electron dot diagram by adding lines between atoms to represent shared pairs in a chemical bond.
In organic chemistry, propyl is a three-carbon alkyl substituent with chemical formula –CH
2CH
2CH
3 for the linear form. This substituent form is obtained by removing one hydrogen atom attached to the terminal carbon of propane. A propyl substituent is often represented in organic chemistry with the symbol Pr.
An anomer is a type of geometric variation found at certain atoms in carbohydrate molecules. An epimer is a stereoisomer that differs in configuration at any single stereogenic center. An anomer is an epimer at the hemiacetal/acetal carbon in a cyclic saccharide, an atom called the anomeric carbon. The anomeric carbon is the carbon derived from the carbonyl carbon of the open-chain form of the carbohydrate molecule. Anomerization is the process of conversion of one anomer to the other. As is typical for stereoisomeric compounds, different anomers have different physical properties, melting points and specific rotations.
In organic chemistry, a locant is a figure to indicate the position of a functional group within a molecule.

In chemistry, a formal charge (FC) is the charge assigned to an atom in a molecule, assuming that electrons in all chemical bonds are shared equally between atoms, regardless of relative electronegativity. When determining the best Lewis structure for a molecule, the structure is chosen such that the formal charge on each of the atoms is as close to zero as possible.
Residual entropy is the difference in entropy between a non-equilibrium state and crystal state of a substance close to absolute zero. This term is used in condensed matter physics to describe the entropy at zero kelvin of a glass or plastic crystal referred to the crystal state, whose entropy is zero according to the third law of thermodynamics. It occurs if a material can exist in many different states when cooled. The most common non-equilibrium state is vitreous state, glass.
An oxygenase is any enzyme that oxidizes a substrate by transferring the oxygen from molecular oxygen O2 (as in air) to it. The oxygenases form a class of oxidoreductases; their EC number is EC 1.13 or EC 1.14.
The chemical substance 1,2-dioxetane (1,2-dioxacyclobutane) is a heterocyclic organic compound with formula C2O2H4, containing a ring of two adjacent oxygen atoms and two adjacent carbon atoms. It is therefore an organic peroxide, and can be viewed as a dimer of formaldehyde (COH2).
1,3-Dioxetane (1,3-dioxacyclobutane) is a heterocyclic organic compound with formula C2O2H4, whose backbone is a four-member ring of alternating oxygen and carbon atoms.

In chemistry, dioxirane is a heterocyclic compound composed of one carbon and two oxygen atoms; it may be thought of as the smallest cyclic organic peroxide. The compound is highly unstable and has never been observed at room temperature. Compounds containing dioxirane as a functional group, called dioxiranes, often possess better stability and are used in organic synthesis as oxidizing reagents, most notably as the key catalytic intermediate in the Shi epoxidation reaction. Other common derivatives employed in organic synthesis include dimethyldioxirane (DMDO) and the more reactive methyl(trifluoromethyl)dioxirane, which are prepared as dilute solutions (~0.1 M) by treatment of acetone and methyl trifluoromethyl ketone, respectively, with Oxone (2KHSO5·KHSO4·K2SO4). Difluorodioxirane, a gas (b.p ~ –80 - –90 °C), is one of the very few dioxirane derivatives that is stable in pure form at room temperature.

The atomic mass (ma) is the mass of an atom. Its unit is the unified atomic mass units where 1 unified atomic mass unit is defined as 1⁄12 of the mass of a single carbon-12 atom, at rest. For atoms, the protons and neutrons of the nucleus account for nearly all of the total mass, and the atomic mass measured in u has nearly the same value as the mass number.








