This page is based on this
Wikipedia article Text is available under the
CC BY-SA 4.0 license; additional terms may apply.
Images, videos and audio are available under their respective licenses.
In the chemical sciences, methylation denotes the addition of a methyl group on a substrate, or the substitution of an atom by a methyl group. Methylation is a form of alkylation, with a methyl group, rather than a larger carbon chain, replacing a hydrogen atom. These terms are commonly used in chemistry, biochemistry, soil science, and the biological sciences.

Histone methyltransferases (HMT) are histone-modifying enzymes, that catalyze the transfer of one, two, or three methyl groups to lysine and arginine residues of histone proteins. The attachment of methyl groups occurs predominantly at specific lysine or arginine residues on histones H3 and H4. Two major types of histone methyltranferases exist, lysine-specific and arginine-specific. In both types of histone methyltransferases, S-Adenosyl methionine (SAM) serves as a cofactor and methyl donor group.
The genomic DNA of eukaryotes associates with histones to form chromatin. The level of chromatin compaction depends heavily on histone methylation and other post-translational modifications of histones. Histone methylation is a principal epigenetic modification of chromatin that determines gene expression, genomic stability, stem cell maturation, cell lineage development, genetic imprinting, DNA methylation, and cell mitosis.
Histone H4 is one of the five main histone proteins involved in the structure of chromatin in eukaryotic cells. Featuring a main globular domain and a long N-terminal tail, H4 is involved with the structure of the nucleosome of the 'beads on a string' organization. Histone proteins are highly post-translationally modified. Covalently bonded modifications include acetylation and methylation of the N-terminal tails. These modifications may alter expression of genes located on DNA associated with its parent histone octamer. Histone H4 is an important protein in the structure and function of chromatin, where its sequence variants and variable modification states are thought to play a role in the dynamic and long term regulation of genes.
Histone methylation is a process by which methyl groups are transferred to amino acids of histone proteins that make up nucleosomes, which the DNA double helix wraps around to form chromosomes. Methylation of histones can either increase or decrease transcription of genes, depending on which amino acids in the histones are methylated, and how many methyl groups are attached. Methylation events that weaken chemical attractions between histone tails and DNA increase transcription, because they enable the DNA to uncoil from nucleosomes so that transcription factor proteins and RNA polymerase can access the DNA. This process is critical for the regulation of gene expression that allows different cells to express different genes.

Methyltransferases are a large group of enzymes that all methylate their substrates but can be split into several subclasses based on their structural features. The most common class of methyltransferases is class I, all of which contain a Rossman fold for binding S-Adenosyl methionine (SAM). Class II methyltransferases contain a SET domain, which are exemplified by SET domain histone methyltransferases, and class III methyltransferases, which are membrane associated. Methyltransferases can also be grouped as different types utilizing different substrates in methyl transfer reactions. These types include protein methyltransferases, DNA/RNA methyltransferases, natural product methyltransferases, and non-SAM dependent methyltransferases. SAM is the classical methyl donor for methyltrasferases, however, examples of other methyl donors are seen in nature. The general mechanism for methyl transfer is a SN2-like nucleophilic attack where the methionine sulfur serves as the nucleophile that transfers the methyl group to the enzyme substrate. SAM is converted to S-Adenosyl homocysteine (SAH) during this process. The breaking of the SAM-methyl bond and the formation of the substrate-methyl bond happen nearly simultaneously. These enzymatic reactions are found in many pathways and are implicated in genetic diseases, cancer, and metabolic diseases.
Histone-arginine N-methyltransferase is an enzyme with systematic name S-adenosyl-L-methionine:histone-arginine Nomega-methyltransferase. This enzyme catalyses the following chemical reaction
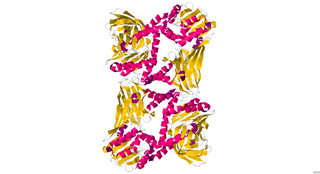
CARM1, also known as PRMT4, is an enzyme encoded by the CARM1 gene found in human beings, as well as many other mammals. It has a polypeptide (L) chain type that is 348 residues long, and is made up of alpha helices and beta sheets. Its main function includes catalyzing the transfer of a methyl group from S-adenosyl-L-methionine to the side chain nitrogens of arginine residues within proteins to form methylated arginine derivatives and S-adenosyl-L-homocysteine. CARM1 is a secondary coactivator through its association with p160 family of coactivators. It is responsible for moving cells toward the inner cell mass in developing blastocysts.
In enzymology, a [cytochrome c]-arginine N-methyltransferase is an enzyme that catalyzes the chemical reaction
In enzymology, a [cytochrome c]-lysine N-methyltransferase (EC 2.1.1.59) is an enzyme that catalyzes the chemical reaction
In enzymology, a [cytochrome-c]-methionine S-methyltransferase is an enzyme that catalyzes the chemical reaction
In enzymology, a [myelin basic protein]-arginine N-methyltransferase is an enzyme that catalyzes the chemical reaction
In enzymology, a [ribulose-bisphosphate carboxylase]-lysine N-methyltransferase (EC 2.1.1.127) is an enzyme that catalyzes the chemical reaction
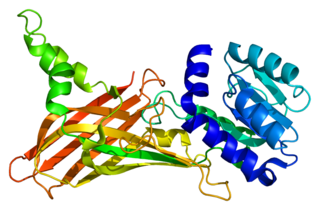
Protein arginine N-methyltransferase 1 is an enzyme that in humans is encoded by the PRMT1 gene.

Euchromatic histone-lysine N-methyltransferase 2 (EHMT2), also known as G9a, is a histone methyltransferase enzyme that in humans is encoded by the EHMT2 gene. G9a catalyzes the mono- and di-methylated states of histone H3 at lysine residue 9 and lysine residue 27.

Protein arginine N-methyltransferase 6 is an enzyme that in humans is encoded by the PRMT6 gene.

The SET domain is a protein domain. It was originally identified as part of a larger conserved region present in the Drosophila Trithorax protein and was subsequently identified in the Drosophila Su(var)3-9 and 'Enhancer of zeste' proteins, from which the acronym SET is derived [Su(var)3-9, Enhancer-of-zeste and Trithorax].
(Fructose-bisphosphate aldolase)-lysine N-methyltransferase (EC 2.1.1.259, rubisco methyltransferase, ribulose-bisphosphate-carboxylase/oxygenase N-methyltransferase, ribulose-1,5-bisphosphate carboxylase/oxygenase large subunit epsilonN-methyltransferase, S-adenosyl-L-methionine:[3-phospho-D-glycerate-carboxy-lyase (dimerizing)]-lysine 6-N-methyltransferase) is an enzyme with systematic name S-adenosyl-L-methionine:(fructose-bisphosphate aldolase)-lysine N6-methyltransferase. This enzyme catalyses the following chemical reaction
Protein methylation is a type of post-translational modification featuring the addition of methyl groups to proteins. It can occur on the nitrogen-containing side-chains of arginine and lysine, but also at the amino- and carboxy-termini of a number of different proteins. In biology, methyltransferases catalyze the methylation process, activated primarily by S-adenosylmethionine.
Protein methylation has been most studied in histones, where the transfer of methyl groups from S-adenosyl methionine is catalyzed by histone methyltransferases. Histones that are methylated on certain residues can act epigenetically to repress or activate gene expression.