
Methionine is an essential amino acid in humans. As the substrate for other amino acids such as cysteine and taurine, versatile compounds such as SAM-e, and the important antioxidant glutathione, methionine plays a critical role in the metabolism and health of many species, including humans. It is encoded by the codon AUG.
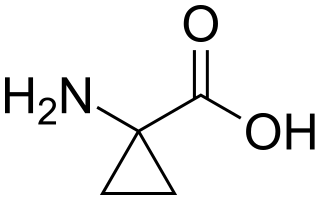
1-Aminocyclopropane-1-carboxylic acid (ACC) is a disubstituted cyclic α-amino acid in which a cyclopropane ring is fused to the Cα atom of the amino acid. It is a white solid. Many cyclopropane-substituted amino acids are known, but this one occurs naturally. Like glycine, but unlike most α-amino acids, ACC is not chiral.

Aminolevulinic acid synthase (ALA synthase, ALAS, or delta-aminolevulinic acid synthase) is an enzyme (EC 2.3.1.37) that catalyzes the synthesis of δ-aminolevulinic acid (ALA) the first common precursor in the biosynthesis of all tetrapyrroles such as hemes, cobalamins and chlorophylls. The reaction is as follows:

Pyridoxal phosphate (PLP, pyridoxal 5'-phosphate, P5P), the active form of vitamin B6, is a coenzyme in a variety of enzymatic reactions. The Enzyme commission has catalogued more than 140 PLP-dependent activities, corresponding to ~4% of all classified activities. The versatility of PLP arises from its ability to covalently bind the substrate, and then to act as an electrophilic catalyst, thereby stabilizing different types of carbanionic reaction intermediates.
Lysine 2,3-aminomutase is a radical SAM enzyme that facilitates the conversion of the amino acid lysine to beta-lysine. It accomplishes this interconversion using three cofactors and a 5'-deoxyadenosyl radical formed in a S-Adenosyl methionine (SAM) activated radical reaction pathway.[1] The generalized reaction is shown below:

Cystathionine-β-synthase, also known as CBS, is an enzyme (EC 4.2.1.22) that in humans is encoded by the CBS gene. It catalyzes the first step of the transsulfuration pathway, from homocysteine to cystathionine:

Cystathionine gamma-lyase is an enzyme which breaks down cystathionine into cysteine, α-ketobutyrate, and ammonia. Pyridoxal phosphate is a prosthetic group of this enzyme.

The transsulfuration pathway is a metabolic pathway involving the interconversion of cysteine and homocysteine through the intermediate cystathionine. Two transsulfurylation pathways are known: the forward and the reverse.
Shang Fa Yang was a Taiwanese-American plant scientist and a professor at the University of California, Davis. He was awarded the Wolf Prize in Agriculture and elected a member of the US National Academy of Sciences.
In enzymology, an aminocyclopropanecarboxylate oxidase (EC 1.14.17.4) is an enzyme that catalyzes the chemical reaction

In enzymology, an alanine racemase is an enzyme that catalyzes the chemical reaction

Cystathionine beta-lyase, also commonly referred to as CBL or β-cystathionase, is an enzyme that primarily catalyzes the following α,β-elimination reaction
In enzymology, a 1-aminocyclopropane-1-carboxylate deaminase (EC 3.5.99.7) is an enzyme that catalyzes the chemical reaction
In enzymology, a discadenine synthase (EC 2.5.1.24) is an enzyme that catalyzes the chemical reaction
In enzymology, an adenosylmethionine-8-amino-7-oxononanoate transaminase is an enzyme that catalyzes the chemical reaction
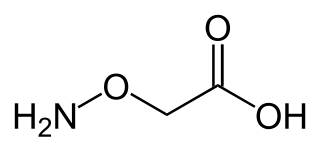
Aminooxyacetic acid, often abbreviated AOA or AOAA, is a compound that inhibits 4-aminobutyrate aminotransferase (GABA-T) activity in vitro and in vivo, leading to less gamma-aminobutyric acid (GABA) being broken down. Subsequently, the level of GABA is increased in tissues. At concentrations high enough to fully inhibit 4-aminobutyrate aminotransferase activity, aminooxyacetic acid is indicated as a useful tool to study regional GABA turnover in rats.

Cobalamin biosynthesis is the process by which bacteria and archea make cobalamin, vitamin B12. Many steps are involved in converting aminolevulinic acid via uroporphyrinogen III and adenosylcobyric acid to the final forms in which it is used by enzymes in both the producing organisms and other species, including humans who acquire it through their diet.

In molecular biology, the Cys/Met metabolism PLP-dependent enzyme family is a family of proteins including enzymes involved in cysteine and methionine metabolism which use PLP (pyridoxal-5'-phosphate) as a cofactor.
Alpha-ketoglutarate-dependent hydroxylases are a major class of non-heme iron proteins that catalyse a wide range of reactions. These reactions include hydroxylation reactions, demethylations, ring expansions, ring closures, and desaturations. Functionally, the αKG-dependent hydroxylases are comparable to cytochrome P450 enzymes. Both use O2 and reducing equivalents as cosubstrates and both generate water.
Ethylene (CH
2=CH
2) is an unsaturated hydrocarbon gas (alkene) acting naturally as a plant hormone. It is the simplest alkene gas and is the first gas known to act as hormone. It acts at trace levels throughout the life of the plant by stimulating or regulating the ripening of fruit, the opening of flowers, the abscission (or shedding) of leaves and, in aquatic and semi-aquatic species, promoting the 'escape' from submergence by means of rapid elongation of stems or leaves. This escape response is particularly important in rice farming. Commercial fruit-ripening rooms use "catalytic generators" to make ethylene gas from a liquid supply of ethanol. Typically, a gassing level of 500 to 2,000 ppm is used, for 24 to 48 hours. Care must be taken to control carbon dioxide levels in ripening rooms when gassing, as high temperature ripening (20 °C; 68 °F) has been seen to produce CO2 levels of 10% in 24 hours.





















