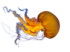
Cnidaria is a phylum under kingdom Animalia containing over 11,000 species of aquatic animals found both in fresh water and marine environments, including jellyfish, hydroids, sea anemones, corals and some of the smallest marine parasites. Their distinguishing features are a decentralized nervous system distributed throughout a gelatinous body and the presence of cnidocytes or cnidoblasts, specialized cells with ejectable flagella used mainly for envenomation and capturing prey. Their bodies consist of mesoglea, a non-living, jelly-like substance, sandwiched between two layers of epithelium that are mostly one cell thick. Cnidarians are also some of the only animals that can reproduce both sexually and asexually.

Invertebrates is an umbrella term describing animals that neither develop nor retain a vertebral column, which evolved from the notochord. It is a paraphyletic grouping including all animals excluding the chordate subphylum Vertebrata, i.e. vertebrates. Well-known phyla of invertebrates include arthropods, mollusks, annelids, echinoderms, flatworms, cnidarians and sponges.

Sponges, the members of the phylum Porifera, are a basal animal clade as a sister of the diploblasts. They are multicellular organisms that have bodies full of pores and channels allowing water to circulate through them, consisting of jelly-like mesohyl sandwiched between two thin layers of cells.

Bilateria is a large clade or infrakingdom of animals called bilaterians, characterized by bilateral symmetry during embryonic development. This means their body plans are laid around a longitudinal axis with a front and a rear end, as well as a left–right–symmetrical belly (ventral) and back (dorsal) surface. Nearly all bilaterians maintain a bilaterally symmetrical body as adults; the most notable exception is the echinoderms, which extend to pentaradial symmetry as adults, but are only bilaterally symmetrical as an embryo. Cephalization is also a characteristic feature among most bilaterians, where the special sense organs and central nerve ganglia become concentrated at the front/rostral end.

Ctenophora comprise a phylum of marine invertebrates, commonly known as comb jellies, that inhabit sea waters worldwide. They are notable for the groups of cilia they use for swimming, and they are the largest animals to swim with the help of cilia.

Ecdysozoa is a group of protostome animals, including Arthropoda, Nematoda, and several smaller phyla. The grouping of these animal phyla into a single clade was first proposed by Eernisse et al. (1992) based on a phylogenetic analysis of 141 morphological characters of ultrastructural and embryological phenotypes. This clade, that is, a group consisting of a common ancestor and all its descendants, was formally named by Aguinaldo et al. in 1997, based mainly on phylogenetic trees constructed using 18S ribosomal RNA genes.

Eumetazoa, also known as diploblasts, Epitheliozoa or Histozoa, are a proposed basal animal clade as a sister group of Porifera (sponges). The basal eumetazoan clades are the Ctenophora and the ParaHoxozoa. Placozoa is now also seen as a eumetazoan in the ParaHoxozoa. The competing hypothesis is the Myriazoa clade.

A nerve net consists of interconnected neurons lacking a brain or any form of cephalization. While organisms with bilateral body symmetry are normally associated with a condensation of neurons or, in more advanced forms, a central nervous system, organisms with radial symmetry are associated with nerve nets, and are found in members of the Ctenophora, Cnidaria, and Echinodermata phyla, all of which are found in marine environments. In the Xenacoelomorpha, a phylum of bilaterally symmetrical animals, members of the subphylum Xenoturbellida also possess a nerve net. Nerve nets can provide animals with the ability to sense objects through the use of the sensory neurons within the nerve net.

Lophotrochozoa is a clade of protostome animals within the Spiralia. The taxon was established as a monophyletic group based on molecular evidence. The clade includes animals like annelids, molluscs, bryozoans, and brachiopods.

Cephalization is an evolutionary trend in animals that, over many generations, the special sense organs and nerve ganglia become concentrated towards the rostral end of the body where the mouth is located, often producing an enlarged head. This is associated with the animal's movement direction and bilateral symmetry, and cephalization of the nervous system led to the formation of a functional centralized brain in three groups of bilaterian animals, namely the arthropods, cephalopod molluscs, and vertebrates (craniates).

Symmetry in biology refers to the symmetry observed in organisms, including plants, animals, fungi, and bacteria. External symmetry can be easily seen by just looking at an organism. For example, the face of a human being has a plane of symmetry down its centre, or a pine cone displays a clear symmetrical spiral pattern. Internal features can also show symmetry, for example the tubes in the human body which are cylindrical and have several planes of symmetry.

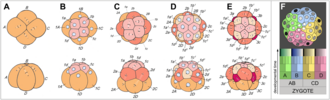
Triploblasty is a condition of the gastrula in which there are three primary germ layers: the ectoderm, mesoderm, and endoderm. Germ cells are set aside in the embryo at the blastula stage, and are incorporated into the gonads during organogenesis. The germ layers form during the gastrulation of the blastula. The term triploblast may refer to any egg cell in which the blastoderm splits into three layers.
Marine life, sea life, or ocean life is the plants, animals, and other organisms that live in the salt water of seas or oceans, or the brackish water of coastal estuaries. At a fundamental level, marine life affects the nature of the planet. Marine organisms, mostly microorganisms, produce oxygen and sequester carbon. Marine life, in part, shape and protect shorelines, and some marine organisms even help create new land.

Marine invertebrates are the invertebrates that live in marine habitats. Invertebrate is a blanket term that includes all animals apart from the vertebrate members of the chordate phylum. Invertebrates lack a vertebral column, and some have evolved a shell or a hard exoskeleton. As on land and in the air, marine invertebrates have a large variety of body plans, and have been categorised into over 30 phyla. They make up most of the macroscopic life in the oceans.

In biology, a phylum is a level of classification or taxonomic rank below kingdom and above class. Traditionally, in botany the term division has been used instead of phylum, although the International Code of Nomenclature for algae, fungi, and plants accepts the terms as equivalent. Depending on definitions, the animal kingdom Animalia contains about 31 phyla, the plant kingdom Plantae contains about 14 phyla, and the fungus kingdom Fungi contains about 8 phyla. Current research in phylogenetics is uncovering the relationships among phyla within larger clades like Ecdysozoa and Embryophyta.
The Cambrian explosion is an interval of time approximately 538.8 million years ago in the Cambrian period of the early Paleozoic when there was a sudden radiation of complex life, and practically all major animal phyla started appearing in the fossil record. It lasted for about 13 to 25 million years and resulted in the divergence of most modern metazoan phyla. The event was accompanied by major diversification in other groups of organisms as well.

Deuterostomes are bilaterian animals of the superphylum Deuterostomia, typically characterized by their anus forming before the mouth during embryonic development. Deuterostomia is further divided into 4 phyla: Chordata, Echinodermata, Hemichordata, and the extinct Vetulicolia known from Cambrian fossils. The extinct clade Cambroernida is also thought to be a member of Deuterostomia.

The Spiralia are a morphologically diverse clade of protostome animals, including within their number the molluscs, annelids, platyhelminths and other taxa. The term Spiralia is applied to those phyla that exhibit canonical spiral cleavage, a pattern of early development found in most members of the Lophotrochozoa.
The evolution of nervous systems dates back to the first development of nervous systems in animals. Neurons developed as specialized electrical signaling cells in multicellular animals, adapting the mechanism of action potentials present in motile single-celled and colonial eukaryotes. Primitive systems, like those found in protists, use chemical signalling for movement and sensitivity; data suggests these were precursors to modern neural cell types and their synapses. When some animals started living a mobile lifestyle and eating larger food particles externally, they developed ciliated epithelia, contractile muscles and coordinating & sensitive neurons for it in their outer layer.

ParaHoxozoa is a clade of animals that consists of Bilateria, Placozoa, and Cnidaria. The relationship of this clade relative to the two other animal lineages Ctenophora and Porifera is debated. Some phylogenomic studies have presented evidence supporting Ctenophora as the sister to Parahoxozoa and Porifera as the sister group to the rest of animals. Other studies have presented evidence supporting Porifera as the sister to Parahoxozoa and Ctenophora as the sister group to the rest of animals, finding that nervous systems either evolved independently in ctenophores and parahoxozoans, or were secondarily lost in poriferans. If ctenophores are taken to have diverged first, Eumetazoa is sometimes used as a synonym for ParaHoxozoa.