This page is based on this
Wikipedia article Text is available under the
CC BY-SA 4.0 license; additional terms may apply.
Images, videos and audio are available under their respective licenses.

Ink is a liquid or paste that contains pigments or dyes and is used to color a surface to produce an image, text, or design. Ink is used for drawing or writing with a pen, brush, or quill. Thicker inks, in paste form, are used extensively in letterpress and lithographic printing.

Deep frying is a cooking method in which food is submerged in hot fat, most commonly oil, rather than the shallow oil used in conventional frying, done in a frying pan. Normally, a deep fryer or chip pan is used for this; industrially, a pressure fryer or vacuum fryer may be used. Deep frying may also be performed using oil that is heated in a pot. Deep frying is classified as hot-fat cooking method. Typically, deep frying foods cook quickly: all sides of a food are cooked simultaneously as oil has a high rate of heat conduction.

A Schiff base (named after Hugo Schiff) is a compound with the general structure R2C=NR' (R' ≠ H). They can be considered a sub-class of imines, being either secondary ketimines or secondary aldimines depending on their structure. The term is often synonymous with azomethine which refers specifically to secondary aldimines (i.e. R-CH=NR' where R' ≠ H).
The Mannich reaction is an organic reaction which consists of an amino alkylation of an acidic proton placed next to a carbonyl functional group by formaldehyde and a primary or secondary amine or ammonia. The final product is a β-amino-carbonyl compound also known as a Mannich base. Reactions between aldimines and α-methylene carbonyls are also considered Mannich reactions because these imines form between amines and aldehydes.
The reaction is named after chemist Carl Mannich.

Ibogaine is a naturally occurring psychoactive substance found in plants in the Apocynaceae family such as Tabernanthe iboga, Voacanga africana and Tabernaemontana undulata. It is a psychedelic with dissociative properties.

The Gassman indole synthesis is a series of chemical reactions used to synthesize substituted indoles by addition of an aniline and a ketone bearing a thioether substituent.
Guaiacol is a naturally-occurring organic compound with the formula C6H4(OH)(OCH3), first isolated by Otto Unverdorben in 1826. Although it is biosynthesized by a variety of organisms, this yellowish aromatic oil is usually derived from guaiacum or wood creosote. Samples darken upon exposure to air and light. Guaiacol is present in wood smoke, resulting from the pyrolysis of lignin. The compound contributes to the flavor of many substances such as whisky and roasted coffee.

o-Toluidine (ortho-toluidine) is an organic compound with the chemical formula CH3C6H4NH2. It is the most important of the three isomeric toluidines. It is a colorless liquid although commercial samples are often yellowish. It is a precursor to the herbicides metolachlor and acetochlor.
Detection of peroxide gives the initial evidence of rancidity in unsaturated fats and oils. Other methods are available, but peroxide value is the most widely used. It gives a measure of the extent to which an oil sample has undergone primary oxidation, extent of secondary oxidation may be determined from p-anisidine test.
The exceptional electrical and mechanical properties of carbon nanotubes have made them alternatives to the traditional electrical actuators for both microscopic and macroscopic applications. Carbon nanotubes are very good conductors of both electricity and heat, and are also very strong and elastic molecules in certain directions. These properties are difficult to find in the same material and very needed for high performance actuators. For current carbon nanotube actuators, multi-walled carbon nanotubes (MWNTs) and bundles of MWNTs have been widely used mostly due to the easiness of handling and robustness. Solution dispersed thick films and highly ordered transparent films of carbon nanotubes have been used for the macroscopic applications.

The total synthesis of the complex biomolecule vitamin B12 was first accomplished by the collaborating research groups of Robert Burns Woodward at Harvard and Albert Eschenmoser at ETH in 1972. It is considered a classic in the field of total synthesis of natural products. Work on the synthesis started 1960 at ETH, and in 1961 at Harvard, it was collaboratively pursued after 1965, and required the effort of 91 post-doctoral fellows (mostly at Harvard) and 12 Ph.D. students (at ETH) from 19 different nations.

2-Carboxybenzaldehyde is a chemical compound. It is an aromatic aldehyde and an aromatic carboxylic acid with ortho-positioned substituents. The drawn 2-formylbenzoic acid is in equilibrium with the cyclic lactol 3-hydroxyphthalide, which forms with alkyl- and aryl-Grignard compounds substituted phthalides. Besides phthalide derivatives, also other benzo-fused heterocycles with different pharmacological properties are derived from 2-carboxybenzaldehyde, for example, isoindolinones or phthalazinones, among them, the antihistamine azelastine.
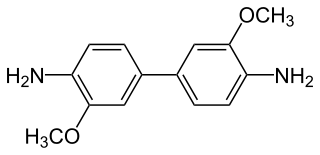
o-Dianisidine is an organic compound with the formula [(CH3O)(H2N)C6H3]2. A colorless or white solid, it is a bifunctional compound derived via the benzidine rearrangement from o-anisidine.

o-Nitroanisole is an organic compound with the formula CH3OC6H4NO2. Three isomers of nitroanisole exist, but the o-isomer is the most commercially important. It is a colorless liquid.











