
A conidium, sometimes termed an asexual chlamydospore or chlamydoconidium, is an asexual, non-motile spore of a fungus. The word conidium comes from the Ancient Greek word for dust, κόνις (kónis). They are also called mitospores due to the way they are generated through the cellular process of mitosis. They are produced exogenously. The two new haploid cells are genetically identical to the haploid parent, and can develop into new organisms if conditions are favorable, and serve in biological dispersal.

Aspergillus terreus, also known as Aspergillus terrestris, is a fungus (mold) found worldwide in soil. Although thought to be strictly asexual until recently, A. terreus is now known to be capable of sexual reproduction. This saprotrophic fungus is prevalent in warmer climates such as tropical and subtropical regions. Aside from being located in soil, A. terreus has also been found in habitats such as decomposing vegetation and dust. A. terreus is commonly used in industry to produce important organic acids, such as itaconic acid and cis-aconitic acid, as well as enzymes, like xylanase. It was also the initial source for the drug mevinolin (lovastatin), a drug for lowering serum cholesterol.

Purpureocillium lilacinum is a species of filamentous fungus in the family Ophiocordycipitaceae. It has been isolated from a wide range of habitats, including cultivated and uncultivated soils, forests, grassland, deserts, estuarine sediments and sewage sludge, and insects. It has also been found in nematode eggs, and occasionally from females of root-knot and cyst nematodes. In addition, it has frequently been detected in the rhizosphere of many crops. The species can grow at a wide range of temperatures – from 8 to 38 °C for a few isolates, with optimal growth in the range 26 to 30 °C. It also has a wide pH tolerance and can grow on a variety of substrates. P. lilacinum has shown promising results for use as a biocontrol agent to control the growth of destructive root-knot nematodes.
Aspergillus ochraceus is a mold species in the genus Aspergillus known to produce the toxin ochratoxin A, one of the most abundant food-contaminating mycotoxins, and citrinin. It also produces the dihydroisocoumarin mellein. It is a filamentous fungus in nature and has characteristic biseriate conidiophores. Traditionally a soil fungus, has now began to adapt to varied ecological niches, like agricultural commodities, farmed animal and marine species. In humans and animals the consumption of this fungus produces chronic neurotoxic, immunosuppressive, genotoxic, carcinogenic and teratogenic effects. Its airborne spores are one of the potential causes of asthma in children and lung diseases in humans. The pig and chicken populations in the farms are the most affected by this fungus and its mycotoxins. Certain fungicides like mancozeb, copper oxychloride, and sulfur have inhibitory effects on the growth of this fungus and its mycotoxin producing capacities.
Aspergillus sydowii is a pathogenic fungus that causes several diseases in humans. It has been implicated in the death of sea fan corals in the Caribbean Sea.
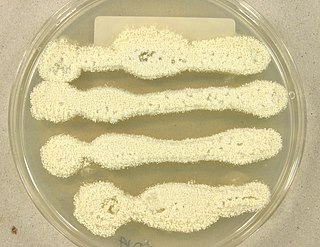
Aspergillus candidus is a white-spored species of fungus in the genus Aspergillus. Despite its lack of pigmentation, it is closely related to the most darkly-pigmented aspergilli in the Aspergillus niger group. It is a common soil fungus worldwide and is known as a contaminant of a wide array of materials from the indoor environment to foods and products. It is an uncommon agent of onychomycosis and aspergillosis. The species epithet candidus (L.) refers to the white pigmentation of colonies of this fungus. It is from the Candidi section. The fungi in the Candidi section are known for their white spores. It has been isolated from wheat flour, djambee, and wheat grain.

Aspergillus glaucus is a filamentous fungus which is known to have a wide environmental distribution due to its physiological hardiness under extreme conditions. Like many other fungi belonging to the genus Aspergillus, it can be mildly pathogenic but has a number of useful potential applications in medicine and the production of foodstuffs.
Emmonsia parva is a filamentous, saprotrophic fungus and one of three species within the genus Emmonsia. The fungus is most known for its causal association with the lung disease, adiaspiromycosis which occurs most commonly in small mammals but is also seen in humans. The disease was first described from rodents in Arizona, and the first human case was reported in France in 1964. Since then, the disease has been reported from Honduras, Brazil, the Czech Republic, Russia, the United States of America and Guatemala. Infections in general are quite rare, especially in humans.

Penicillium digitatum is a mesophilic fungus found in the soil of citrus-producing areas. It is a major source of post-harvest decay in fruits and is responsible for the widespread post-harvest disease in Citrus fruit known as green rot or green mould. In nature, this necrotrophic wound pathogen grows in filaments and reproduces asexually through the production of conidiophores and conidia. However, P. digitatum can also be cultivated in the laboratory setting. Alongside its pathogenic life cycle, P. digitatum is also involved in other human, animal and plant interactions and is currently being used in the production of immunologically based mycological detection assays for the food industry.
Aspergillus unguis is a species of fungus in the genus Aspergillus, and the asexual state (anamorph) of Emericella unguis. Aspergillus unguis is a filamentous soil-borne fungus found on decomposing plant matter and other moist substrates including with building materials and household dust. Aspergillus unguis occurs mainly in tropical and subtropical soils but has also been isolated from various marine and aquatic habitats. The species was first isolated in 1935 by Weill and L. Gaudin. Historically, A. unguis was assigned to the A. nidulans group, a common group of soil-borne fungi due to the resemblance of its ascospores and cleistothecia to those of Emericella nidulans. Aspergillus unguis is distinctive, however, in possessing spicular hyphae. A number of synonyms have been collapsed into this species, including Sterigmatocystis unguis, Aspergillus laokiashanensis and Aspergillus mellinus.

Trichothecium roseum is a fungus in the division Ascomycota first reported in 1809. It is characterized by its flat and granular colonies which are initially white and develop to be light pink in color. This fungus reproduces asexually through the formation of conidia with no known sexual state. Trichothecium roseum is distinctive from other species of the genus Trichothecium in its characteristic zigzag patterned chained conidia. It is found in various countries worldwide and can grow in a variety of habitats ranging from leaf litter to fruit crops. Trichothecium roseum produces a wide variety of secondary metabolites including mycotoxins, such as roseotoxins and trichothecenes, which can infect and spoil a variety of fruit crops. It can act as both a secondary and opportunistic pathogen by causing pink rot on various fruits and vegetables and thus has an economical impact on the farming industry. Secondary metabolites of T. roseum, specifically Trichothecinol A, are being investigated as potential anti-metastatic drugs. Several agents including harpin, silicon oxide, and sodium silicate are potential inhibitors of T. roseum growth on fruit crops. Trichothecium roseum is mainly a plant pathogen and has yet to show a significant impact on human health.

Cladosporium cladosporioides is a darkly pigmented mold that occurs world-wide on a wide range of materials both outdoors and indoors. It is one of the most common fungi in outdoor air where its spores are important in seasonal allergic disease. While this species rarely causes invasive disease in animals, it is an important agent of plant disease, attacking both the leaves and fruits of many plants. This species produces asexual spores in delicate, branched chains that break apart readily and drift in the air. It is able to grow under low water conditions and at very low temperatures.
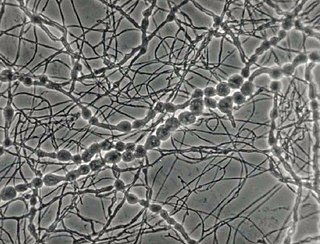
Madurella mycetomatis is a fungus primarily reported in Central Africa as a cause of mycetoma in humans. It has been misclassified for many years, but with improvement of molecular techniques, its phylogenetic classification has been established. Many methods exist to identify M. mycetomatis, both in lesions and in culture. Histological examination is especially useful, as it has many unique morphological features. Strain-level differences in response to antifungal agents is informative for treatment and laboratory isolation of cultures.
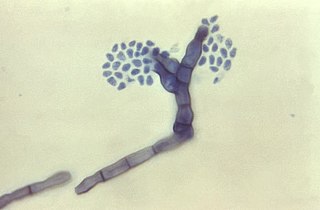
Fonsecaea compacta is a saprophytic fungal species found in the family Herpotrichiellaceae. It is a rare etiological agent of chromoblastomycosis, with low rates of correspondence observed from reports. The main active components of F. compacta are glycolipids, yet very little is known about its composition. F. compacta is widely regarded as a dysplastic variety of Fonsecaea pedrosoi, its morphological precursor. The genus Fonsecaea presently contains two species, F. pedrosoi and F. compacta. Over 100 strains of F. pedrosoi have been isolated but only two of F. compacta.

Aspergillus alabamensis is a soil fungus in the division Ascomycota first described in 2009 as a segregated taxon of A. terreus. Originally thought to be a variant of A. terreus, A. alabamensis is situated in a distinctive clade identified by genetic analysis. While A. alabamensis has been found to be morphologically similar to Aspergillus terreus by morphological studies, the two differ significantly in active metabolic pathways, with A. alabamensis producing the mycotoxins citrinin and citreoviridin but lacking mevinolin.

Aspergillus parasiticus is a fungus belonging to the genus Aspergillus. This species is an unspecialized saprophytic mold, mostly found outdoors in areas of rich soil with decaying plant material as well as in dry grain storage facilities. Often confused with the closely related species, A. flavus, A. parasiticus has defined morphological and molecular differences. Aspergillus parasiticus is one of three fungi able to produce the mycotoxin, aflatoxin, one of the most carcinogenic naturally occurring substances. Environmental stress can upregulate aflatoxin production by the fungus, which can occur when the fungus is growing on plants that become damaged due to exposure to poor weather conditions, during drought, by insects, or by birds. In humans, exposure to A. parasiticus toxins can cause delayed development in children and produce serious liver diseases and/or hepatic carcinoma in adults. The fungus can also cause the infection known as aspergillosis in humans and other animals. A. parasiticus is of agricultural importance due to its ability to cause disease in corn, peanut, and cottonseed.

Penicillium spinulosum is a non-branched, fast-growing fungus with a swelling at the terminal of the stipe (vesiculate) in the genus Penicillium. P. spinulosum is able to grow and reproduce in environment with low temperature and low water availability, and is known to be acidotolerant. P. spinulosum is ubiquitously distributed, and can often be isolated from soil. Each individual strain of P. spinulosum differs from others in their colony morphology, including colony texture, amount of sporulation and roughness of conidia and conidiophores.
Aspergillus wentii is an asexual, filamentous, endosymbiotic fungus belonging to the mold genus, Aspergillus. It is a common soil fungus with a cosmopolitan distribution, although it is primarily found in subtropical regions. Found on a variety of organic materials, A. wentii is known to colonize corn, cereals, moist grains, peanuts and other ground nut crops. It is also used in the manufacture of biodiesel from lipids and is known for its ability to produce enzymes used in the food industry.

Mariannaea elegans an anamorphic fungus. It is mainly found on rotting wood and soil. M. elegans is not pathogenic to humans, animals, or plants.
Aspergillus giganteus is a species of fungus in the genus Aspergillus that grows as a mold. It was first described in 1901 by Wehmer, and is one of six Aspergillus species from the Clavati section of the subgenus Fumigati. Its closest taxonomic relatives are Aspergillus rhizopodus and Aspergillus longivescia.















