A neurotransmitter is a signaling molecule secreted by a neuron to affect another cell across a synapse. The cell receiving the signal, or target cell, may be another neuron, but could also be a gland or muscle cell.

Monoamine neurotransmitters are neurotransmitters and neuromodulators that contain one amino group connected to an aromatic ring by a two-carbon chain (such as -CH2-CH2-). Examples are dopamine, norepinephrine and serotonin.

Reuptake is the reabsorption of a neurotransmitter by a neurotransmitter transporter located along the plasma membrane of an axon terminal or glial cell after it has performed its function of transmitting a neural impulse.

Monoamine transporters (MATs) are proteins that function as integral plasma-membrane transporters to regulate concentrations of extracellular monoamine neurotransmitters. The three major classes are serotonin transporters (SERTs), dopamine transporters (DATs), and norepinephrine transporters (NETs) and are responsible for the reuptake of their associated amine neurotransmitters. MATs are located just outside the synaptic cleft (peri-synaptically), transporting monoamine transmitter overflow from the synaptic cleft back to the cytoplasm of the pre-synaptic neuron. MAT regulation generally occurs through protein phosphorylation and post-translational modification. Due to their significance in neuronal signaling, MATs are commonly associated with drugs used to treat mental disorders as well as recreational drugs. Compounds targeting MATs range from medications such as the wide variety of tricyclic antidepressants, selective serotonin reuptake inhibitors such as fluoxetine (Prozac) to stimulant medications such as methylphenidate (Ritalin) and amphetamine in its many forms and derivatives methamphetamine (Desoxyn) and lisdexamfetamine (Vyvanse). Furthermore, drugs such as MDMA and natural alkaloids such as cocaine exert their effects in part by their interaction with MATs, by blocking the transporters from mopping up dopamine, serotonin, and other neurotransmitters from the synapse.

Serotonin–norepinephrine reuptake inhibitors (SNRIs) are a class of antidepressant medications used to treat major depressive disorder (MDD), anxiety disorders, social phobia, chronic neuropathic pain, fibromyalgia syndrome (FMS), and menopausal symptoms. Off-label uses include treatments for attention-deficit hyperactivity disorder (ADHD), obsessive–compulsive disorder (OCD), and migraine prevention. SNRIs are monoamine reuptake inhibitors; specifically, they inhibit the reuptake of serotonin and norepinephrine. These neurotransmitters are thought to play an important role in mood regulation. SNRIs can be contrasted with the selective serotonin reuptake inhibitors (SSRIs) and norepinephrine reuptake inhibitors (NRIs), which act upon single neurotransmitters.
The vesicular monoamine transporter (VMAT) is a transport protein integrated into the membranes of synaptic vesicles of presynaptic neurons. It transports monoamine neurotransmitters – such as dopamine, serotonin, norepinephrine, epinephrine, and histamine – into the vesicles, which release the neurotransmitters into synapses, as chemical messages to postsynaptic neurons. VMATs utilize a proton gradient generated by V-ATPases in vesicle membranes to power monoamine import.

Selegiline, also known as L-deprenyl and sold under the brand names Eldepryl, Emsam, Selgin, among other names, is a medication which is used in the treatment of Parkinson's disease and major depressive disorder. It is provided in the form of a capsule or tablet taken by mouth or orally disintegrating tablets taken on the tongue for Parkinson's disease and as a patch applied to skin for depression.

Dopaminergic means "related to dopamine" (literally, "working on dopamine"), dopamine being a common neurotransmitter. Dopaminergic substances or actions increase dopamine-related activity in the brain. Dopaminergic brain pathways facilitate dopamine-related activity. For example, certain proteins such as the dopamine transporter (DAT), vesicular monoamine transporter 2 (VMAT2), and dopamine receptors can be classified as dopaminergic, and neurons that synthesize or contain dopamine and synapses with dopamine receptors in them may also be labeled as dopaminergic. Enzymes that regulate the biosynthesis or metabolism of dopamine such as aromatic L-amino acid decarboxylase or DOPA decarboxylase, monoamine oxidase (MAO), and catechol O-methyl transferase (COMT) may be referred to as dopaminergic as well. Also, any endogenous or exogenous chemical substance that acts to affect dopamine receptors or dopamine release through indirect actions (for example, on neurons that synapse onto neurons that release dopamine or express dopamine receptors) can also be said to have dopaminergic effects, two prominent examples being opioids, which enhance dopamine release indirectly in the reward pathways, and some substituted amphetamines, which enhance dopamine release directly by binding to and inhibiting VMAT2.

The norepinephrine transporter (NET), also known as noradrenaline transporter (NAT), is a protein that in humans is encoded by the solute carrier family 6 member 2 (SLC6A2) gene.

Levomethamphetamine is the levorotatory (L-enantiomer) form of methamphetamine. Levomethamphetamine is a sympathomimetic vasoconstrictor that is the active ingredient in some over-the-counter (OTC) nasal decongestant inhalers in the United States.

Trace amines are an endogenous group of trace amine-associated receptor 1 (TAAR1) agonists – and hence, monoaminergic neuromodulators – that are structurally and metabolically related to classical monoamine neurotransmitters. Compared to the classical monoamines, they are present in trace concentrations. They are distributed heterogeneously throughout the mammalian brain and peripheral nervous tissues and exhibit high rates of metabolism. Although they can be synthesized within parent monoamine neurotransmitter systems, there is evidence that suggests that some of them may comprise their own independent neurotransmitter systems.

Rasagiline is an irreversible inhibitor of monoamine oxidase-B used as a monotherapy to treat symptoms in early Parkinson's disease or as an adjunct therapy in more advanced cases.
A serotonin–norepinephrine–dopamine reuptake inhibitor (SNDRI), also known as a triple reuptake inhibitor (TRI), is a type of drug that acts as a combined reuptake inhibitor of the monoamine neurotransmitters serotonin, norepinephrine, and dopamine. It does this by concomitantly inhibiting the serotonin transporter (SERT), norepinephrine transporter (NET), and dopamine transporter (DAT), respectively. Inhibition of the reuptake of these neurotransmitters increases their extracellular concentrations and, therefore, results in an increase in serotonergic, adrenergic, and dopaminergic neurotransmission. The naturally-occurring and potent SNDRI cocaine is widely used recreationally and often illegally for the euphoric effects it produces.

(-)-1-Phenyl-2-propylaminopentane is a stimulant of the substituted phenethylamine class that has been derived from selegiline. When compared with selegiline and other substituted phenethylamines (-)-PPAP has a notably different mechanism of action and pharmacological effect.

Reuptake inhibitors (RIs) are a type of reuptake modulators. It is a drug that inhibits the plasmalemmal transporter-mediated reuptake of a neurotransmitter from the synapse into the pre-synaptic neuron. This leads to an increase in extracellular concentrations of the neurotransmitter and an increase in neurotransmission. Various drugs exert their psychological and physiological effects through reuptake inhibition, including many antidepressants and psychostimulants.

A monoamine releasing agent (MRA), or simply monoamine releaser, is a drug that induces the release of a monoamine neurotransmitter from the presynaptic neuron into the synapse, leading to an increase in the extracellular concentrations of the neurotransmitter. Many drugs induce their effects in the body and/or brain via the release of monoamine neurotransmitters, e.g., trace amines, many substituted amphetamines, and related compounds.
A serotonin releasing agent (SRA) is a type of drug that induces the release of serotonin into the neuronal synaptic cleft. A selective serotonin releasing agent (SSRA) is an SRA with less significant or no efficacy in producing neurotransmitter efflux at other types of monoamine neurons.
A monoamine reuptake inhibitor (MRI) is a drug that acts as a reuptake inhibitor of one or more of the three major monoamine neurotransmitters serotonin, norepinephrine, and dopamine by blocking the action of one or more of the respective monoamine transporters (MATs), which include the serotonin transporter (SERT), norepinephrine transporter (NET), and dopamine transporter (DAT). This in turn results in an increase in the synaptic concentrations of one or more of these neurotransmitters and therefore an increase in monoaminergic neurotransmission.
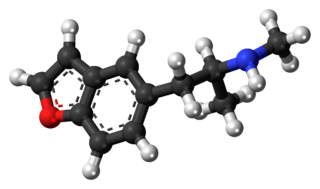
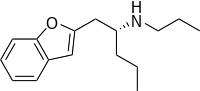
5-MAPB is an entactogenic designer drug similar to MDMA in its structure and effects.
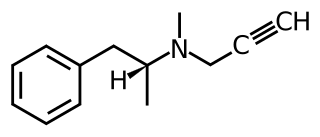
Monoamine activity enhancers (MAE), also known as catecholaminergic/serotoninergic activity enhancers, are a class neuro-biologically active compounds that enhance the release of monoamines in the nervous system. Monoamine activity enhancers are distinct from monoamine releasing agents in that they do not cause the release of monoamines from synaptic vesicles but rather potentiate impulse-evoked monoamine-release. Monoamine activity enhancers increase the number of monoamines released per electrical impulse received.