Ecology
Marine

The Black Sea supports an active and dynamic marine ecosystem, dominated by species suited to the brackish, nutrient-rich, conditions. As with all marine food webs, the Black Sea features a range of trophic groups, with autotrophic algae, including diatoms and dinoflagellates, acting as primary producers. The fluvial systems draining Eurasia and central Europe introduce large volumes of sediment and dissolved nutrients into the Black Sea, but the distribution of these nutrients is controlled by the degree of physiochemical stratification, which is, in turn, dictated by seasonal physiographic development. [7]
During winter, strong wind promotes convective overturning and upwelling of nutrients, while high summer temperatures result in a marked vertical stratification and a warm, shallow mixed layer. [8] Day length and insolation intensity also controls the extent of the photic zone. Subsurface productivity is limited by nutrient availability, as the anoxic bottom waters act as a sink for reduced nitrate, in the form of ammonia. The benthic zone also plays an important role in Black Sea nutrient cycling, as chemosynthetic organisms and anoxic geochemical pathways recycle nutrients which can be upwelled to the photic zone, enhancing productivity. [9]
In total, Black Sea's biodiversity contains around one-third of Mediterranean's and is experiencing natural and artificial invasions or Mediterranizations. [10] [11]
Phytoplankton

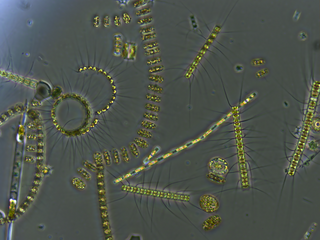
The main phytoplankton groups present in the Black Sea are dinoflagellates, diatoms, coccolithophores and cyanobacteria. Generally, the annual cycle of phytoplankton development comprises significant diatom and dinoflagellate-dominated spring production, followed by a weaker mixed assemblage of community development below the seasonal thermocline during summer months and surface-intensified autumn production. [8] [12] This pattern of productivity is also augmented by an Emiliania huxleyi bloom during the late spring and summer months.
- Annual dinoflagellate distribution is defined by an extended bloom period in subsurface waters during the late spring and summer. In November, subsurface plankton production is combined with surface production, due to vertical mixing of water masses and nutrients such as nitrite. [7] The major bloom-forming dinoflagellate species in the Black Sea is Gymnodinium sp. [13] Estimates of dinoflagellate diversity in the Black Sea range from 193 [14] to 267 species. [15] This level of species richness is relatively low in comparison to the Mediterranean Sea, which is attributable to the brackish conditions, low water transparency and presence of anoxic bottom waters. It is also possible that the low winter temperatures below 4 °C (39 °F) of the Black Sea prevent thermophilous species from becoming established. The relatively high organic matter content of Black Sea surface water favor the development of heterotrophic (an organism that uses organic carbon for growth) and mixotrophic dinoflagellates species (able to exploit different trophic pathways), relative to autotrophs. Despite its unique hydrographic setting, there are no confirmed endemic dinoflagellate species in the Black Sea. [15]
- The Black Sea is populated by many species of the marine diatom, which commonly exist as colonies of unicellular, non-motile auto- and heterotrophic algae. The life-cycle of most diatoms can be described as 'boom and bust' and the Black Sea is no exception, with diatom blooms occurring in surface waters throughout the year, most reliably during March. [7] In simple terms, the phase of rapid population growth in diatoms is caused by the in-wash of silicon-bearing terrestrial sediments, and when the supply of silicon is exhausted, the diatoms begin to sink out of the photic zone and produce resting cysts. Additional factors such as predation by zooplankton and ammonium-based regenerated production also have a role to play in the annual diatom cycle. [7] [8] Typically, Proboscia alata blooms during spring and Pseudosolenia calcar-avis blooms during the autumn. [13]
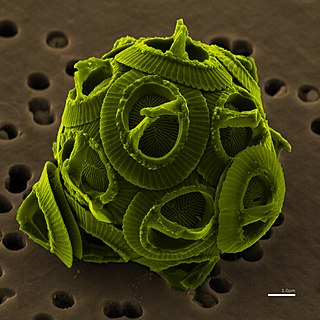
- Coccolithophores are a type of motile, autotrophic phytoplankton that produce CaCO3 plates, known as coccoliths, as part of their life cycle. In the Black Sea, the main period of coccolithophore growth occurs after the bulk of the dinoflagellate growth has taken place. In May, the dinoflagellates move below the seasonal thermocline, into deeper waters, where more nutrients are available. This permits coccolithophores to utilize the nutrients in the upper waters, and by the end of May, with favorable light and temperature conditions, growth rates reach their highest. The major bloom-forming species is Emiliania huxleyi , which is also responsible for the release of dimethyl sulfide into the atmosphere. Overall, coccolithophore diversity is low in the Black Sea, and although recent sediments are dominated by E. huxleyi, Braarudosphaera bigelowii, Holocene sediments have also been shown to contain Helicopondosphaera and Discolithina species.
- Cyanobacteria are a phylum of picoplanktonic (plankton ranging in size from 0.2 to 2.0 µm) bacteria that obtain their energy via photosynthesis, and are present throughout the world's oceans. They exhibit a range of morphologies, including filamentous colonies and biofilms. In the Black Sea, several species are present, and as an example, Synechococcus spp. can be found throughout the photic zone, although concentration decreases with increasing depth. Other factors which exert an influence on distribution include nutrient availability, predation, and salinity. [16]
Animal species
- The Black Sea along with the Caspian Sea is part of the Zebra mussel's native range. The mussel has been accidentally introduced around the world and become an invasive species where it has been introduced.
- The Common Carp's native range extends to The Black Sea along with the Caspian Sea and Aral Sea. Like the Zebra mussel the Common Carp is an invasive species when introduced to other habitats.
- Is another native fish that is also found in the Caspian Sea. It preys upon Zebra mussels. Like the mussels and common carp it has become invasive when introduced to other environments, like the Great Lakes.
- Marine Mammals and marine megafaunas
- Marine mammals present within the basin include two species of dolphins (common [17] and bottlenose [18] ) and harbour porpoise [19] inhabit the sea although all of these are endangered due to pressures and impacts by human activities. All the three species have been classified as a distinct subspecies from those in the Mediterranean and in Atlantic Seas and endemic to Black and Azov Seas, and are more active during nights in Turkish Straits. [20] However, construction of the Crimean Bridge caused increases in nutrients and planktons in the waters, attracting large numbers of fish and more than 1,000 bottlenose dolphins. [21] On the other hand, however, others claim that construction may cause devastating damages on ecosystem including dolphins. [22]
- Critically endangered Mediterranean monk seals were historically abundant in Black Sea, and are regarded to have become extinct from the basin in 1997. [23] Monk seals were present at the Snake Island until 1950s, and several locations such as the Danube Plavni Nature Reserve and Doğankent were last of hauling-out sites in post-1990. [24] Very few animals still thrive in the Sea of Marmara. [25]
- Ongoing Mediterranizations may or may not boost in increases of cetacean diversity in Turkish Straits [20] hence in Black and Azov basins.
- Various species of pinnipeds, sea otter, and beluga whales [26] [27] were introduced into the Black Sea by mankind and later escaped either by accidental or purported causes. Of these, grey seal [28] and beluga whales [26] have been recorded with successful, long-term occurrences.
- Great white sharks are known to reach into the Sea of Marmara and Bosporus Strait and basking shark into Dardanelles although it is unclear whether or not these sharks may reach into the Black and Azov basins. [29] [30]
Ecological effects of pollution
Since the 1960s, rapid industrial expansion along the Black Sea coast line and the construction of a major dam has significantly increased annual variability in the N:P:Si ratio in the basin. In coastal areas, the biological effect of these changes has been an increase in the frequency of monospecific phytoplankton blooms, with diatom bloom frequency increasing by a factor of 2.5 and non-diatom bloom frequency increasing by a factor of 6. The non-diatoms, such as the prymnesiophytes Emiliania huxleyi (coccolithophore), Chromulina sp., and the Euglenophyte Eutreptia lanowii are able to out-compete diatom species because of the limited availability of Si, a necessary constituent of diatom frustules. [31] As a consequence of these blooms, benthic macrophyte populations were deprived of light, while anoxia caused mass mortality in marine animals. [32] [33]
The decline in macrophytes was further compounded by overfishing during the 1970s, while the invasive ctenophore Mnemiopsis reduced the biomass of copepods and other zooplankton in the late 1980s. Additionally, an alien species—the warty comb jelly (Mnemiopsis leidyi)—was able to establish itself in the basin, exploding from a few individuals to estimated biomass of one billion metric tons. [34] The change in species composition in Black Sea waters also has consequences for hydrochemistry, as Ca-producing coccolithophores influence salinity and pH, although these ramifications have yet to be fully quantified. In central Black Sea waters, Si levels were also significantly reduced, due to a decrease in the flux of Si associated with advection across isopycnal surfaces. This phenomenon demonstrates the potential for localized alterations in Black Sea nutrient input to have basin-wide effects.
Pollution reduction and regulation efforts have led to a partial recovery of the Black Sea ecosystem during the 1990s, and an EU monitoring exercise, 'EROS21', revealed decreased N and P values, relative to the 1989 peak. [35] Recently, scientists have noted signs of ecological recovery, in part due to the construction of new sewage treatment plants in Slovakia, Hungary, Romania, and Bulgaria in connection with membership in the European Union. Mnemiopsis leidyi populations have been checked with the arrival of another alien species which feeds on them. [36]