
Boron nitride is a thermally and chemically resistant refractory compound of boron and nitrogen with the chemical formula BN. It exists in various crystalline forms that are isoelectronic to a similarly structured carbon lattice. The hexagonal form corresponding to graphite is the most stable and soft among BN polymorphs, and is therefore used as a lubricant and an additive to cosmetic products. The cubic variety analogous to diamond is called c-BN; it is softer than diamond, but its thermal and chemical stability is superior. The rare wurtzite BN modification is similar to lonsdaleite but slightly softer than the cubic form.

Beta carbon nitride (β-C3N4) is a superhard material predicted to be harder than diamond.

Silicon carbide (SiC), also known as carborundum, is a semiconductor containing silicon and carbon. It occurs in nature as the extremely rare mineral moissanite. Synthetic SiC powder has been mass-produced since 1893 for use as an abrasive. Grains of silicon carbide can be bonded together by sintering to form very hard ceramics that are widely used in applications requiring high endurance, such as car brakes, car clutches and ceramic plates in bulletproof vests. Electronic applications of silicon carbide such as light-emitting diodes (LEDs) and detectors in early radios were first demonstrated around 1907. SiC is used in semiconductor electronics devices that operate at high temperatures or high voltages, or both. Large single crystals of silicon carbide can be grown by the Lely method and they can be cut into gems known as synthetic moissanite.
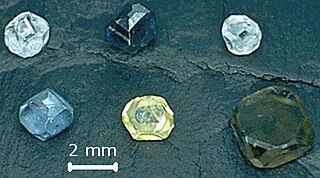
Synthetic diamond is a diamond made of the same material as natural diamonds: pure carbon, crystallized in an isotropic 3D form. Synthetic diamonds are different from both natural diamond, which is created by geological processes, and diamond simulant, which is made of non-diamond material.
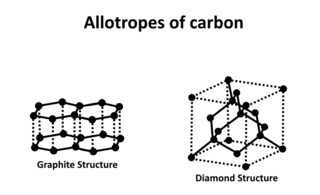
Carbon is capable of forming many allotropes due to its valency. Well-known forms of carbon include diamond and graphite. In recent decades, many more allotropes have been discovered and researched including ball shapes such as buckminsterfullerene and sheets such as graphene. Larger scale structures of carbon include nanotubes, nanobuds and nanoribbons. Other unusual forms of carbon exist at very high temperatures or extreme pressures. Around 500 hypothetical 3-periodic allotropes of carbon are known at the present time, according to the Samara Carbon Allotrope Database (SACADA).
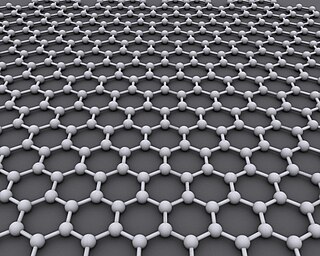
Graphene is an allotrope of carbon consisting of a single layer of atoms arranged in a two-dimensional honeycomb lattice. The name is a portmanteau of "graphite" and the suffix -ene, reflecting the fact that the graphite allotrope of carbon consists of stacked graphene layers.
Amorphous carbon is free, reactive carbon that does not have any crystalline structure. Amorphous carbon materials may be stabilized by terminating dangling-π bonds with hydrogen. As with other amorphous solids, some short-range order can be observed. Amorphous carbon is often abbreviated to aC for general amorphous carbon, aC:H or HAC for hydrogenated amorphous carbon, or to ta-C for tetrahedral amorphous carbon.

Aluminium nitride (AlN) is a solid nitride of aluminium. It has a high thermal conductivity of up to 321 W/(m·K) and is an electrical insulator. Its wurtzite phase (w-AlN) has a band gap of ~6 eV at room temperature and has a potential application in optoelectronics operating at deep ultraviolet frequencies.
A superhard material is a material with a hardness value exceeding 40 gigapascals (GPa) when measured by the Vickers hardness test. They are virtually incompressible solids with high electron density and high bond covalency. As a result of their unique properties, these materials are of great interest in many industrial areas including, but not limited to, abrasives, polishing and cutting tools, disc brakes, and wear-resistant and protective coatings.

Diamond is the allotrope of carbon in which the carbon atoms are arranged in the specific type of cubic lattice called diamond cubic. Diamond is crystal that is transparent to opaque and which is generally isotropic. Diamond is the hardest naturally occurring material known. Yet, due to important structural weaknesses, diamond's toughness is only fair to good. The precise tensile strength of bulk diamond is unknown; however, compressive strength up to 60 GPa has been observed, and it could be as high as 90–100 GPa in the form of nanometer-sized wires or needles ,with a corresponding local maximum tensile elastic strain in excess of 9%. The anisotropy of diamond hardness is carefully considered during diamond cutting. Diamond has a high refractive index (2.417) and moderate dispersion (0.044) properties that give cut diamonds their brilliance. Scientists classify diamonds into four main types according to the nature of crystallographic defects present. Trace impurities substitutionally replacing carbon atoms in a diamond's crystal structure, and in some cases structural defects, are responsible for the wide range of colors seen in diamond. Most diamonds are electrical insulators and extremely efficient thermal conductors. Unlike many other minerals, the specific gravity of diamond crystals (3.52) has rather small variation from diamond to diamond.

Imperfections in the crystal lattice of diamond are common. Such crystallographic defects in diamond may be the result of lattice irregularities or extrinsic substitutional or interstitial impurities, introduced during or after the diamond growth. The defects affect the material properties of diamond and determine to which type a diamond is assigned; the most dramatic effects are on the diamond color and electrical conductivity, as explained by the electronic band structure.

Aggregated diamond nanorods, or ADNRs, are a nanocrystalline form of diamond, also known as nanodiamond or hyperdiamond.

Carbon nanofibers (CNFs), vapor grown carbon fibers (VGCFs), or vapor grown carbon nanofibers (VGCNFs) are cylindrical nanostructures with graphene layers arranged as stacked cones, cups or plates. Carbon nanofibers with graphene layers wrapped into perfect cylinders are called carbon nanotubes.
Nanochemistry is the combination of chemistry and nano science. Nanochemistry is associated with synthesis of building blocks which are dependent on size, surface, shape and defect properties. Nanochemistry is being used in chemical, materials and physical, science as well as engineering, biological and medical applications. Nanochemistry and other nanoscience fields have the same core concepts but the usages of those concepts are different.

The nitrogen-vacancy center is one of numerous point defects in diamond. Its most explored and useful property is photoluminescence, which can be easily detected from an individual NV center, especially those in the negative charge state (NV−). Electron spins at NV centers, localized at atomic scales, can be manipulated at room temperature by applying a magnetic field, electric field, microwave radiation or light, or a combination, resulting in sharp resonances in the intensity and wavelength of the photoluminescence. These resonances can be explained in terms of electron spin related phenomena such as quantum entanglement, spin-orbit interaction and Rabi oscillations, and analysed using advanced quantum optics theory. An individual NV center can be viewed as a basic unit of a quantum computer, and it has potential applications in novel, more efficient fields of electronics and computational science including quantum cryptography, spintronics, and masers. If the charge is not specified the term "NV center" refers to the negatively charged NV− center.
A microplasma is a plasma of small dimensions, ranging from tens to thousands of micrometers. Microplasmas can be generated at a variety of temperatures and pressures, existing as either thermal or non-thermal plasmas. Non-thermal microplasmas that can maintain their state at standard temperatures and pressures are readily available and accessible to scientists as they can be easily sustained and manipulated under standard conditions. Therefore, they can be employed for commercial, industrial, and medical applications, giving rise to the evolving field of microplasmas.

Nanodiamonds or diamond nanoparticles are diamonds with a size below 1 micrometre. They can be produced by impact events such as an explosion or meteoritic impacts. Because of their inexpensive, large-scale synthesis, potential for surface functionalization, and high biocompatibility, nanodiamonds are widely investigated as a potential material in biological and electronic applications and quantum engineering.
Nitrogen-doped carbon nanotubes (N-CNTs) can be produced through five main methods; chemical vapor deposition, high-temperature and high-pressure reactions, gas-solid reaction of amorphous carbon with NH3 at high temperature, solid reaction, and solvothermal synthesis.
Although diamonds on Earth are rare, extraterrestrial diamonds are very common. Diamonds so tiny that they contain only about 2000 carbon atoms are abundant in meteorites and some of them formed in stars before the Solar System existed. High pressure experiments suggest large amounts of diamonds are formed from methane on the ice giant planets Uranus and Neptune, while some planets in other planetary systems may be almost pure diamond. Diamonds are also found in stars and may have been the first mineral ever to have formed.

Jagdish Narayan is an Indian-born American engineer. Since 2001, he has served as the John C. C. Fan Family Distinguished Chair Professor in the Materials Science and Engineering Department at North Carolina State University. He is also the distinguished visiting scientist at Oak Ridge National Laboratory. Narayan has published above 500 high-impact journal articles, with his discoveries covered in over 40 US and international patents. His body of work can be segregated into highly nonequilibrium laser processing of novel nanomaterials, including Q-carbon, Q-BN, diamond and c-BN related materials. These research articles have received over 31,000 Google Citations with h-index >85. Narayan and his students discovered Q-carbon as the new allotrope, thereby finding a new route to fabricate diamond and related materials in ambient conditions, resulting in properties and applications ranging from high-temperature superconductivity in Boron-doped Q-carbon to hardness than diamond in Q-carbon to enhanced field-emission in Q-carbon to Nitrogen-doped nanodiamonds for quantum computing, nanosensing and solid-state devices.
















