Related Research Articles
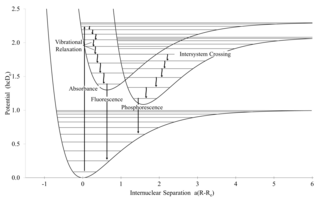
Intersystem crossing (ISC) is an isoenergetic radiationless process involving a transition between the two electronic states with different spin multiplicity.

Cyclobutadiene is an organic compound with the formula C4H4. It is very reactive owing to its tendency to dimerize. Although the parent compound has not been isolated, some substituted derivatives are robust and a single molecule of cyclobutadiene is quite stable. Since the compound degrades by a bimolecular process, the species can be observed by matrix isolation techniques at temperatures below 35 K. It is thought to adopt a rectangular structure.
In organic chemistry, a carbene is a molecule containing a neutral carbon atom with a valence of two and two unshared valence electrons. The general formula is R−:C−R' or R=C: where the R represents substituents or hydrogen atoms.
A non-Kekulé molecule is a conjugated hydrocarbon that cannot be assigned a classical Kekulé structure.
In chemistry, a nitrene or imene is the nitrogen analogue of a carbene. The nitrogen atom is uncharged and univalent, so it has only 6 electrons in its valence level—two covalent bonded and four non-bonded electrons. It is therefore considered an electrophile due to the unsatisfied octet. A nitrene is a reactive intermediate and is involved in many chemical reactions. The simplest nitrene, HN, is called imidogen, and that term is sometimes used as a synonym for the nitrene class.

Sulfur monoxide is an inorganic compound with formula SO. It is only found as a dilute gas phase. When concentrated or condensed, it converts to S2O2 (disulfur dioxide). It has been detected in space but is rarely encountered intact otherwise.

Singlet oxygen, systematically named dioxygen(singlet) and dioxidene, is a gaseous inorganic chemical with the formula O=O, which is in a quantum state where all electrons are spin paired. It is kinetically unstable at ambient temperature, but the rate of decay is slow.

Triplet oxygen, 3O2, refers to the S = 1 electronic ground state of molecular oxygen (dioxygen). It is the most stable and common allotrope of oxygen. Molecules of triplet oxygen contain two unpaired electrons, making triplet oxygen an unusual example of a stable and commonly encountered diradical: it is more stable as a triplet than a singlet. According to molecular orbital theory, the electron configuration of triplet oxygen has two electrons occupying two π molecular orbitals (MOs) of equal energy (that is, degenerate MOs). In accordance with Hund's rules, they remain unpaired and spin-parallel and account for the paramagnetism of molecular oxygen. These half-filled orbitals are antibonding in character, reducing the overall bond order of the molecule to 2 from a maximum value of 3 (e.g., dinitrogen), which occurs when these antibonding orbitals remain fully unoccupied. The molecular term symbol for triplet oxygen is 3Σ−
g.
Spin chemistry is a sub-field of chemistry and physics, positioned at the intersection of chemical kinetics, photochemistry, magnetic resonance and free radical chemistry, that deals with magnetic and spin effects in chemical reactions. Spin chemistry concerns phenomena such as chemically induced dynamic nuclear polarization (CIDNP), chemically induced electron polarization (CIDEP), magnetic isotope effects in chemical reactions, and it is hypothesized to be key in the underlying mechanism for avian magnetoreception and consciousness.

Atomic carbon, systematically named carbon and λ0-methane, also called monocarbon, is a colourless gaseous inorganic chemical with the chemical formula C. It is kinetically unstable at ambient temperature and pressure, being removed through autopolymerisation.
In spectroscopy and quantum chemistry, the multiplicity of an energy level is defined as 2S+1, where S is the total spin angular momentum. States with multiplicity 1, 2, 3, 4, 5 are respectively called singlets, doublets, triplets, quartets and quintets.
In chemistry, a (redox) non-innocent ligand is a ligand in a metal complex where the oxidation state is not clear. Typically, complexes containing non-innocent ligands are redox active at mild potentials. The concept assumes that redox reactions in metal complexes are either metal or ligand localized, which is a simplification, albeit a useful one.
An electronic effect influences the structure, reactivity, or properties of molecule but is neither a traditional bond nor a steric effect. In organic chemistry, the term stereoelectronic effect is also used to emphasize the relation between the electronic structure and the geometry (stereochemistry) of a molecule.

In chemistry, a radical, also known as a free radical, is an atom, molecule, or ion that has at least one unpaired valence electron. With some exceptions, these unpaired electrons make radicals highly chemically reactive. Many radicals spontaneously dimerize. Most organic radicals have short lifetimes.
Spin states when describing transition metal coordination complexes refers to the potential spin configurations of the central metal's d electrons. For several oxidation states, metals can adopt high-spin and low-spin configurations. The ambiguity only applies to first row metals, because second- and third-row metals are invariably low-spin. These configurations can be understood through the two major models used to describe coordination complexes; crystal field theory and ligand field theory.
Singlet fission is a spin-allowed process, unique to molecular photophysics, whereby one singlet excited state is converted into two triplet states. The phenomenon has been observed in molecular crystals, aggregates, disordered thin films, and covalently-linked dimers, where the chromophores are oriented such that the electronic coupling between singlet and the double triplet states is large. Being spin allowed, the process can occur very rapidly and out-compete radiative decay thereby producing two triplets with very high efficiency. The process is distinct from intersystem crossing, in that singlet fission does not involve a spin flip, but is mediated by two triplets coupled into an overall singlet. It has been proposed that singlet fission in organic photovoltaic devices could improve the photoconversion efficiencies.

9-Fluorenylidene is an aryl carbene derived from the bridging methylene group of fluorene. Fluorenylidene has the unusual property that the triplet ground state is only 1.1 kcal/mol lower in energy than the singlet state. For this reason, fluorenylidene has been studied extensively in organic chemistry.

Plumbylenes (or plumbylidenes) are divalent organolead(II) analogues of carbenes, with the general chemical formula, R2Pb, where R denotes a substituent. Plumbylenes possess 6 electrons in their valence shell, and are considered open shell species.

Nickel bis(stilbenedithiolate) or bis(dithiobenzil)nickel is a coordination complex with the formula Ni(S2C2Ph2)2 (where Ph = phenyl). It exists as a black solid that gives green solutions in toluene due to a strong absorption at 855 nm. The complex is a prototype of a large family of bis(dithiolene) complexes or the formula Ni(S2C2R2)2 (R = H, alkyl, aryl). These complexes have attracted much attention as dyes. They are of academic interest because the dithiolenes are noninnocent ligands. The lengths of the C-S and C-C bonds in the backbone, respectively 1.71 and 1.39 Å, are intermediate between double and single bonds.

1,3-Diphospha-2,4-diboretanes, or B2P2, is a class of 4-member cyclic compounds of alternating boron and phosphorus atoms. They are often found as dimers during the synthesis of boraphosphenes (RB=PR'). Compounds can exhibit localized singlet diradical character (diradicaloid) between the boron atoms in the solution and solid state.
References
- ↑ "Diradicals" (pdf). Gold Book. IUPAC. 2014. doi:10.1351/goldbook.D01765.
- ↑ Abe M (September 2013). "Diradicals". Chemical Reviews. 113 (9): 7011–7088. doi:10.1021/cr400056a. PMID 23883325.
- 1 2 Aragoni MC, Caltagirone C, Lippolis V, Podda E, Slawin AM, Woollins JD, et al. (December 2020). "Diradical Character of Neutral Heteroleptic Bis(1,2-dithiolene) Metal Complexes: Case Study of [Pd(Me2timdt)(mnt)] (Me2timdt = 1,3-Dimethyl-2,4,5-trithioxoimidazolidine; mnt2- = 1,2-Dicyano-1,2-ethylenedithiolate)". Inorganic Chemistry. 59 (23): 17385–17401. doi:10.1021/acs.inorgchem.0c02696. PMC 7735710 . PMID 33185438.
- ↑ Ray K, Weyhermüller T, Neese F, Wieghardt K (July 2005). "Electronic structure of square planar bis(benzene-1,2-dithiolato)metal complexes [M(L)(2)](z) (z = 2-, 1-, 0; M = Ni, Pd, Pt, Cu, Au): an experimental, density functional, and correlated ab initio study". Inorganic Chemistry. 44 (15): 5345–5360. doi:10.1021/ic0507565. PMID 16022533.
- ↑ "Triplet State". Gold Book. IUPAC. 2014. doi:10.1351/goldbook.T06503.
- ↑ Bachler V, Olbrich G, Neese F, Wieghardt K (August 2002). "Theoretical evidence for the singlet diradical character of square planar nickel complexes containing two o-semiquinonato type ligands". Inorganic Chemistry. 41 (16): 4179–4193. doi:10.1021/ic0113101. PMID 12160406.
- ↑ Sharma, Mahendra K.; Ebeler, Falk; Glodde, Timo; Neumann, Beate; Stammler, Hans-Georg; Ghadwal, Rajendra S. (2021-01-13). "Isolation of a Ge(I) Diradicaloid and Dihydrogen Splitting". Journal of the American Chemical Society. 143 (1): 121–125. doi:10.1021/jacs.0c11828. ISSN 0002-7863. PMID 33373236. S2CID 229719653.
- ↑ Seenithurai S, Chai JD (July 2017). "Effect of Li Termination on the Electronic and Hydrogen Storage Properties of Linear Carbon Chains: A TAO-DFT Study". Scientific Reports. 7 (1): 4966. arXiv: 1702.03055 . Bibcode:2017NatSR...7.4966S. doi:10.1038/s41598-017-05202-6. PMC 5504039 . PMID 28694445.