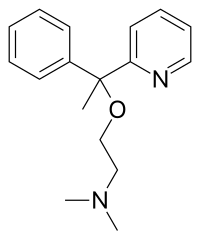
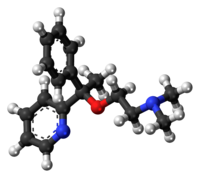
Hypnotic, or soporific drugs, commonly known as sleeping pills, are a class of psychoactive drugs whose primary function is to induce sleep and to treat insomnia (sleeplessness).
H1 antagonists, also called H1 blockers, are a class of medications that block the action of histamine at the H1 receptor, helping to relieve allergic reactions. Agents where the main therapeutic effect is mediated by negative modulation of histamine receptors are termed antihistamines; other agents may have antihistaminergic action but are not true antihistamines.
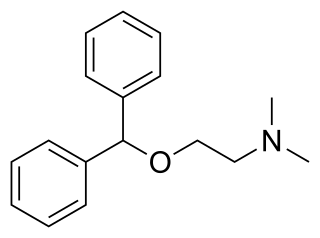
Diphenhydramine (DPH) is an antihistamine and sedative mainly used to treat allergies, insomnia, and symptoms of the common cold. It is also less commonly used for tremors in parkinsonism, and nausea. It is taken by mouth, injected into a vein, injected into a muscle, or applied to the skin. Maximal effect is typically around two hours after a dose, and effects can last for up to seven hours.

Benadryl is a brand of various antihistamine medications used to stop allergies, whose content varies in different countries, but which includes some combination of diphenhydramine, acrivastine, and/or cetirizine.

Hydroxyzine, sold under the brand names Atarax and Vistaril among others, is an antihistamine medication. It is used in the treatment of itchiness, insomnia, anxiety, and nausea, including that due to motion sickness. It is used either by mouth or injection into a muscle.
Vicks DayQuil is an over-the-counter combination medication product used for the temporary relief of common cold and flu symptoms. DayQuil is available in several formulations.

Cetirizine is a second-generation antihistamine used to treat allergic rhinitis, dermatitis, and urticaria (hives). It is taken by mouth. Effects generally begin within thirty minutes and last for about a day. The degree of benefit is similar to other antihistamines such as diphenhydramine, which is a first-generation antihistamine.
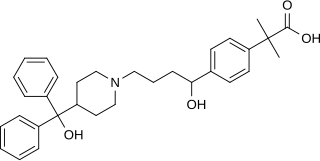
Fexofenadine, sold under the brand name Allegra among others, is an antihistamine pharmaceutical drug used in the treatment of allergy symptoms, such as hay fever and urticaria.

Promethazine, sold under the brand name Phenergan among others, is a first-generation antihistamine, antipsychotic, sedative, and antiemetic used to treat allergies, insomnia, and nausea. It may also help with some symptoms associated with the common cold and may also be used for sedating people who are agitated or anxious, an effect that has led to some recreational use. Promethazine is taken by mouth (oral), as a rectal suppository, or by injection into a muscle (IM).

Clemastine, also known as meclastin, is a first-generation H1 histamine antagonist (antihistamine) with anticholinergic properties (drying) and sedative side effects. Like all first-generation antihistamines, it is sedating.

Doxepin is a medication belonging to the tricyclic antidepressant (TCA) class of drugs used to treat major depressive disorder, anxiety disorders, chronic hives, and insomnia. For hives it is a less preferred alternative to antihistamines. It has a mild to moderate benefit for sleeping problems. It is used as a cream for itchiness due to atopic dermatitis or lichen simplex chronicus.

Trimipramine, sold under the brand name Surmontil among others, is a tricyclic antidepressant (TCA) which is used to treat depression. It has also been used for its sedative, anxiolytic, and weak antipsychotic effects in the treatment of insomnia, anxiety disorders, and psychosis, respectively. The drug is described as an atypical or "second-generation" TCA because, unlike other TCAs, it seems to be a fairly weak monoamine reuptake inhibitor. Similarly to other TCAs however, trimipramine does have antihistamine, antiserotonergic, antiadrenergic, antidopaminergic, and anticholinergic activities.
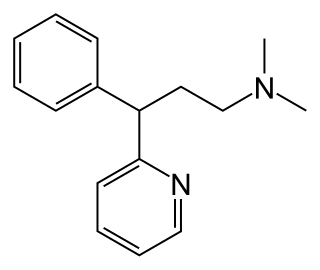
Pheniramine is an antihistamine with anticholinergic properties used to treat allergic conditions such as hay fever or urticaria. It has relatively strong sedative effects, and may sometimes be used off-label as an over-the-counter sleeping pill in a similar manner to other sedating antihistamines such as diphenhydramine. Pheniramine is also commonly found in eyedrops used for the treatment of allergic conjunctivitis.
Pyridoxine/doxylamine, sold under the brand name Diclectin among others, is a combination of pyridoxine hydrochloride (vitamin B6) and doxylamine succinate. It is generally used for nausea and vomiting of pregnancy (morning sickness); even though its efficacy has not been proven and subsequent research has led to the removal of recommendations in medical journals.

Tripelennamine, sold under the brand name Pyribenzamine by Novartis, is a drug that is used as an antipruritic and first-generation antihistamine. It can be used in the treatment of asthma, hay fever, rhinitis, and urticaria, but is now less common as it has been replaced by newer antihistamines. The drug was patented at CIBA, which merged with Geigy into Ciba-Geigy, and eventually becoming Novartis.
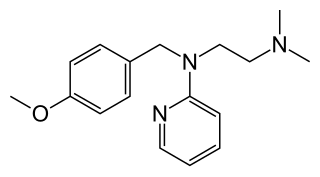
Mepyramine, also known as pyrilamine, is a first generation antihistamine, targeting the H1 receptor as an inverse agonist. Mepyramine rapidly permeates the brain, often causing drowsiness. It is often sold as a maleate salt, pyrilamine maleate.

Antihistamines are drugs which treat allergic rhinitis, common cold, influenza, and other allergies. Typically, people take antihistamines as an inexpensive, generic drug that can be bought without a prescription and provides relief from nasal congestion, sneezing, or hives caused by pollen, dust mites, or animal allergy with few side effects. Antihistamines are usually for short-term treatment. Chronic allergies increase the risk of health problems which antihistamines might not treat, including asthma, sinusitis, and lower respiratory tract infection. Consultation of a medical professional is recommended for those who intend to take antihistamines for longer-term use.

Suvorexant, sold under the brand name Belsomra, is an orexin antagonist medication which is used in the treatment of insomnia. It is indicated specifically for the treatment of insomnia characterized by difficulties with sleep onset and/or maintenance in adults. Suvorexant helps with falling asleep faster, sleeping longer, being awake less in the middle of the night, and having better quality of sleep. Its effectiveness is modest, and is similar to that of other orexin antagonists, but is lower than that of benzodiazepines and Z-drugs. Suvorexant is taken by mouth.
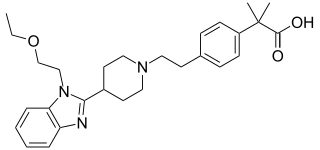
Bilastine is an antihistamine medication used to treat hives (urticaria), allergic rhinitis and itchy inflamed eyes (allergic conjunctivitis) caused by an allergy. It is a second-generation antihistamine and takes effect by selectively inhibiting the histamine H1 receptor, preventing these allergic reactions. Bilastine has an effectiveness similar to cetirizine, fexofenadine, and desloratadine.

Somnifacient, also known as sedatives or sleeping pills, is a class of medications that induces sleep. It is mainly used for treatment of insomnia. Examples of somnifacients include benzodiazepines, barbiturates and antihistamines.