A bronchodilator is a substance that dilates the bronchi and bronchioles, decreasing resistance in the respiratory airway and increasing airflow to the lungs. Bronchodilators may be endogenous, or they may be medications administered for the treatment of breathing difficulties. They are most useful in obstructive lung diseases, of which asthma and chronic obstructive pulmonary disease are the most common conditions. Although this remains somewhat controversial, they might be useful in bronchiolitis and bronchiectasis. They are often prescribed but of unproven significance in restrictive lung diseases.
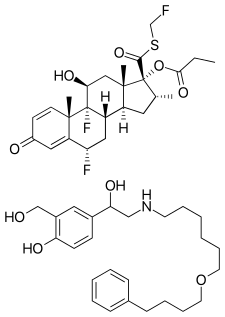
Budesonide/formoterol, sold under the brand name Symbicort among others, is a combination medication used in the management of asthma or chronic obstructive pulmonary disease (COPD). It contains budesonide, a steroid and formoterol, a long-acting β2-agonist (LABA). It is not recommended for sudden worsening or treatment of active bronchospasm. It is used by breathing in the medication.

Formoterol, also known as eformoterol, is a long-acting β2 agonist (LABA) used as a bronchodilator in the management of asthma and COPD. Formoterol has an extended duration of action compared to short-acting β2 agonists such as salbutamol (albuterol), which are effective for 4 h to 6 h. LABAs such as formoterol are used as "symptom controllers" to supplement prophylactic corticosteroid therapy. A "reliever" short-acting β2 agonist is still required, since LABAs are not recommended for the treatment of acute asthma.
β2 (beta2) adrenergic receptor agonists, also known as adrenergic β2 receptor agonists, are a class of drugs that act on the β2 adrenergic receptor. Like other β adrenergic agonists, they cause smooth muscle relaxation. β2 adrenergic agonists' effects on smooth muscle cause dilation of bronchial passages, vasodilation in muscle and liver, relaxation of uterine muscle, and release of insulin. They are primarily used to treat asthma and other pulmonary disorders, such as COPD.

Bronchoconstriction is the constriction of the airways in the lungs due to the tightening of surrounding smooth muscle, with consequent coughing, wheezing, and shortness of breath.
Long-acting β adrenoceptor agonists are usually prescribed for moderate-to-severe persistent asthma patients or patients with chronic obstructive pulmonary disease (COPD). They are designed to reduce the need for shorter-acting β2 agonists such as salbutamol (albuterol), as they have a duration of action of approximately 12 hours in comparison with the 4-to-6-hour duration of salbutamol, making them candidates for sparing high doses of corticosteroids or treating nocturnal asthma and providing symptomatic improvement in patients with COPD. With the exception of formoterol, long-acting β2 agonists are not recommended for the treatment of acute asthma exacerbations because of their slower onset of action compared to salbutamol. Their long duration of action is due to the addition of a long, lipophilic side-chain that binds to an exosite on adrenergic receptors. This allows the active portion of the molecule to continuously bind and unbind at β2 receptors in the smooth muscle in the lungs.

Mometasone, also known as mometasone furoate, is a steroid medication used to treat certain skin conditions, hay fever, and asthma. Specifically it is used to prevent rather than treat asthma attacks. It can be applied to the skin, inhaled, or used in the nose.
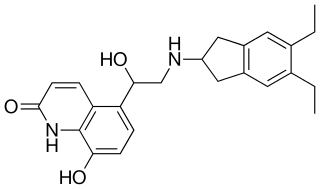
Indacaterol (INN) is an ultra-long-acting beta-adrenoceptor agonist developed by Novartis. It was approved by the European Medicines Agency (EMA) under the trade name Onbrez Breezhaler on November 30, 2009, and by the United States Food and Drug Administration (FDA), under the trade name Arcapta Neohaler, on July 1, 2011. It needs to be taken only once a day, unlike the related drugs formoterol and salmeterol. It is licensed only for the treatment of chronic obstructive pulmonary disease (COPD). It is delivered as an aerosol formulation through a dry powder inhaler.
Fluticasone furoate is a corticosteroid for the treatment of non-allergic and allergic rhinitis administered by a nasal spray. It is derived from cortisol.

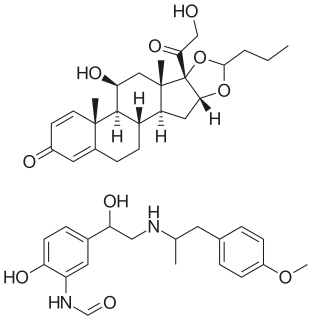
Mometasone/formoterol is a combination inhaler containing both an inhaled corticosteroid and long-acting bronchodilator. It is indicated for the maintenance treatment of asthma in adults and children 12 years of age and older whose asthma is not well controlled on low- or medium-dose corticosteroids, or whose disease clearly warrants combination therapy. It is not approved for the treatment of acute bronchospasm. To relieve acute symptoms, a rapid-onset short-duration inhaled bronchodilator should be available to the patient for use.

Olodaterol is an ultra-long-acting β adrenoreceptor agonist (ultra-LABA) used as an inhalation for treating patients with chronic obstructive pulmonary disease (COPD), manufactured by Boehringer Ingelheim.

Vilanterol is an ultra-long-acting β2 adrenoreceptor agonist (ultra-LABA), which was approved in May 2013 in combination with fluticasone furoate for sale as Breo Ellipta by GlaxoSmithKline for the treatment of chronic obstructive pulmonary disease (COPD).

Fluticasone furoate/vilanterol (FF/VI), sold under the trade names Breo Ellipta among others, is a combination medication for the treatment of chronic obstructive pulmonary disease (COPD) and asthma. It contains fluticasone furoate, an inhaled corticosteroid, and vilanterol, an ultra-long-acting β2 agonist (ultra-LABA).
β2-adrenoceptor agonists is a group of drugs that act selectively on β2-receptors in the lungs causing bronchodilation. β2-agonists are used to treat asthma and COPD, diseases that cause obstruction in the airways. The first β2-agonist isoproterenol, an unselective agonist which was discovered in the 1940s. The aim of the drug development through the years has been to minimise side effects, achieve selectivity and longer duration of action. The mechanism of action is well understood and has facilitated the development. The structure of the binding site and the nature of the binding is also well known, as is the structure activity relationship.

Indacaterol/glycopyrronium bromide is a combination drug for inhalation consisting of the following two active ingredients:

Carmoterol(INN), also known as TA-2005 and CHF-4226, is a non-catechol experimental ultra-long-acting β adrenoreceptor agonist (ultra-LABA) that was in clinical trials before 2010 when it has been withdrawn from further development based on evidence that the compound does not possess a competitive profile.