
The Food and Drug Administration is a federal agency of the United States Department of Health and Human Services, one of the United States federal executive departments. The FDA is responsible for protecting and promoting public health through the control and supervision of food safety, tobacco products, dietary supplements, prescription and over-the-counter pharmaceutical drugs (medications), vaccines, biopharmaceuticals, blood transfusions, medical devices, electromagnetic radiation emitting devices (ERED), cosmetics, animal foods & feed and veterinary products.

The Pure Food and Drug Act of 1906 was the first of a series of significant consumer protection laws which was enacted by Congress in the 20th century and led to the creation of the Food and Drug Administration. Its main purpose was to ban foreign and interstate traffic in adulterated or mislabeled food and drug products, and it directed the U.S. Bureau of Chemistry to inspect products and refer offenders to prosecutors. It required that active ingredients be placed on the label of a drug's packaging and that drugs could not fall below purity levels established by the United States Pharmacopeia or the National Formulary. The Jungle by Upton Sinclair with its graphic and revolting descriptions of unsanitary conditions and unscrupulous practices rampant in the meatpacking industry, was an inspirational piece that kept the public's attention on the important issue of unhygienic meat processing plants that later led to food inspection legislation. Sinclair quipped, "I aimed at the public's heart and by accident I hit it in the stomach," as outraged readers demanded and got the pure food law.
Anticonvulsants are a diverse group of pharmacological agents used in the treatment of epileptic seizures. Anticonvulsants are also increasingly being used in the treatment of bipolar disorder and borderline personality disorder, since many seem to act as mood stabilizers, and for the treatment of neuropathic pain. Anticonvulsants suppress the excessive rapid firing of neurons during seizures. Anticonvulsants also prevent the spread of the seizure within the brain.

A generic drug is a pharmaceutical drug that contains the same chemical substance as a drug that was originally protected by chemical patents. Generic drugs are allowed for sale after the patents on the original drugs expire. Because the active chemical substance is the same, the medical profile of generics is believed to be equivalent in performance. A generic drug has the same active pharmaceutical ingredient (API) as the original, but it may differ in some characteristics such as the manufacturing process, formulation, excipients, color, taste, and packaging.
The pregnancy category of a medication is an assessment of the risk of fetal injury due to the pharmaceutical, if it is used as directed by the mother during pregnancy. It does not include any risks conferred by pharmaceutical agents or their metabolites in breast milk.

Good manufacturing practices (GMP) are the practices required in order to conform to the guidelines recommended by agencies that control the authorization and licensing of the manufacture and sale of food and beverages, cosmetics, pharmaceutical products, dietary supplements, and medical devices. These guidelines provide minimum requirements that a manufacturer must meet to assure that their products are consistently high in quality, from batch to batch, for their intended use. The rules that govern each industry may differ significantly; however, the main purpose of GMP is always to prevent harm from occurring to the end user. Additional tenets include ensuring the end product is free from contamination, that it is consistent in its manufacture, that its manufacture has been well documented, that personnel are well trained, and the product has been checked for quality more than just at the end phase. GMP is typically ensured through the effective use of a quality management system (QMS).

Generally recognized as safe (GRAS) is a United States Food and Drug Administration (FDA) designation that a chemical or substance added to food is considered safe by experts, and so is exempted from the usual Federal Food, Drug, and Cosmetic Act (FFDCA) food additive tolerance requirements. The concept of food additives being "generally recognized as safe" was first described in the Food Additives Amendment of 1958, and all additives introduced after this time had to be evaluated by new standards.

Cannabidiol (CBD) is a phytocannabinoid discovered in 1940. It is one of 113 identified cannabinoids in cannabis plants and accounts for up to 40% of the plant's extract. In 2018, clinical research on cannabidiol included preliminary studies of anxiety, cognition, movement disorders, and pain.

The United States Federal Food, Drug, and Cosmetic Act, is a set of laws passed by Congress in 1938 giving authority to the U.S. Food and Drug Administration (FDA) to oversee the safety of food, drugs, medical devices, and cosmetics. A principal author of this law was Royal S. Copeland, a three-term U.S. Senator from New York. In 1968, the Electronic Product Radiation Control provisions were added to the FD&C. Also in that year the FDA formed the Drug Efficacy Study Implementation (DESI) to incorporate into FD&C regulations the recommendations from a National Academy of Sciences investigation of effectiveness of previously marketed drugs. The act has been amended many times, most recently to add requirements about bioterrorism preparations.
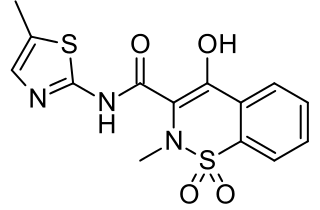
Meloxicam, sold under the brand name Mobic among others, is a nonsteroidal anti-inflammatory drug (NSAID) used to treat pain and inflammation in rheumatic diseases and osteoarthritis. It is taken by mouth. It is recommended that it be used for as short a period as possible and at a low dose.
Health food is a marketing term to suggest human health effects beyond a normal healthy diet required for human nutrition. Foods marketed as health foods may be part of one or more categories, such as natural foods, organic foods, whole foods, vegetarian foods or dietary supplements. These products may be sold in health food stores or in the health food or organic sections of grocery stores. While there is no precise definition for "health food", the United States Food and Drug Administration monitors and warns food manufacturers against labeling foods as having specific health effects when no evidence exists to support such statements.

Losartan, sold under the trade name Cozaar among others, is a medication mainly used to treat high blood pressure. It is also used for diabetic kidney disease, heart failure, and left ventricular enlargement. It is taken by mouth. It may be used alone or in addition to other blood pressure medication. Up to six weeks may be required for the full effects to occur.

The National Medical Products Administration (NMPA) was founded on the basis of the former State Food and Drug Administration (SFDA). In March 2013, the former regulatory body was rebranded and restructured as the China Food and Drug Administration, elevating it to a ministerial-level agency. In 2018, as part of China's 2018 government administration overhaul, the name was changed to 'National Medical Products Administration' and merged into the newly-created State Administration for Market Regulation. The headquarters are in Xicheng District, Beijing.
Iontocaine was an anesthetic medication, marketed under the two brand names Numby and Phoresor PM900 by IOMED inc. It is a local anesthetic with vasoconstrictor, administered via iontophoresis through the skin. It can numb up to 10 mm of skin in as little as 10 minutes. It is a 2% lidocaine, 0.01 mg/ml epinephrine solution. It was manufactured by IOMED, Inc.
Title 21 is the portion of the Code of Federal Regulations that governs food and drugs within the United States for the Food and Drug Administration (FDA), the Drug Enforcement Administration (DEA), and the Office of National Drug Control Policy (ONDCP).

Eugeroics, also known as wakefulness-promoting agents and wakefulness-promoting drugs, are a class of drugs that promote wakefulness and alertness. They are used medically for the treatment of certain sleeping disorders, such as excessive daytime sleepiness and narcolepsy. They generally have a very low addictive potential.
The Ministry of Food and Drug Safety, formerly known as the Korea Food & Drug Administration, is a South Korea government agency responsible for promoting the public health by ensuring the safety and efficiency of foods, pharmaceuticals, medical devices and cosmetics as well as supporting the development of the food and pharmaceutical industries. The main goal is to offer people safe foods and drugs.

Solriamfetol, sold under the brand name Sunosi, is a medication used for the treatment of excessive sleepiness associated with narcolepsy and sleep apnea.
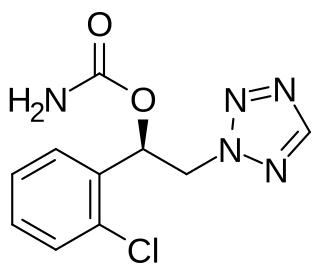
Cenobamate, sold under the brand name Xcopri, is for the treatment of partial-onset seizures in adults.










