This page is based on this
Wikipedia article Text is available under the
CC BY-SA 4.0 license; additional terms may apply.
Images, videos and audio are available under their respective licenses.
A reducing agent is an element or compound that loses an electron to another chemical species in a redox chemical reaction. Since the reducing agent is losing electrons, it is said to have been oxidized.
In chemistry, a reactivity series (or activity series) is an empirical, calculated, and structurally analytical progression of a series of metals, arranged by their "reactivity" from highest to lowest. It is used to summarize information about the reactions of metals with acids and water, double displacement reactions and the extraction of metals from their ores.

Titanium tetrachloride is the inorganic compound with the formula TiCl4. It is an important intermediate in the production of titanium metal and the pigment titanium dioxide. TiCl4 is a volatile liquid. Upon contact with humid air, it forms spectacular opaque clouds of titanium dioxide (TiO2) and hydrated hydrogen chloride. It is sometimes referred to as "tickle" or "tickle 4" due to the phonetic resemblance of its molecular formula (TiCl4) to the word.

Tin(II) chloride, also known as stannous chloride, is a white crystalline solid with the formula SnCl2. It forms a stable dihydrate, but aqueous solutions tend to undergo hydrolysis, particularly if hot. SnCl2 is widely used as a reducing agent (in acid solution), and in electrolytic baths for tin-plating. Tin(II) chloride should not be confused with the other chloride of tin; tin(IV) chloride or stannic chloride (SnCl4).

Akaganeite, also written as the deprecated Akaganéite, is a chloride-containing iron(III) oxide-hydroxide mineral, formed by the weathering of pyrrhotite (Fe1−xS).

Iron(III) oxide-hydroxide or ferric oxyhydroxide is the chemical compound of iron, oxygen, and hydrogen with formula FeO(OH).
Hydrochloric acid regeneration or HCl regeneration refers to a chemical process for the reclamation of bound and unbound HCl from metal chloride solutions such as hydrochloric acid.
iron(II) carbonate, or ferrous carbonate, is a chemical compound with formula FeCO
3, that occurs naturally as the mineral siderite. At ordinary ambient temperatures, it is a green-brown ionic solid consisting of iron(II) cations Fe2+
and carbonate anions CO2−
3.

Hydrochloric acid or muriatic acid is a colorless inorganic chemical system with the formula H
2O:HCl. Hydrochloric acid has a distinctive pungent smell. It is classified as strongly acidic and can attack the skin over a wide composition range, since the hydrogen chloride completely dissociates in aqueous solution.
Iron(III) sulfide, also known as ferric sulfide or sesquisulfide, is one of the three iron sulfides besides FeS and FeS2. It is a solid, black powder but decays at ambient temperature into a yellow-green powder.
Stock nomenclature for inorganic compounds is a widely used system of chemical nomenclature developed by the German chemist Alfred Stock and first published in 1919. In the "Stock system", the oxidation states of some or all of the elements in a compound are indicated in parentheses by Roman numerals.
Iron oxychloride is the inorganic compound with the formula FeOCl. This purple solid adopts a layered structure, akin to that of cadmium chloride. The material slowly hydrolyses in moist air. The solid intercalates electron donors such as tetrathiafulvalene and even pyridine to give mixed valence charge-transfer salts. Intercalation is accompanied by a marked increase in electrical conductivity and a color change to black.
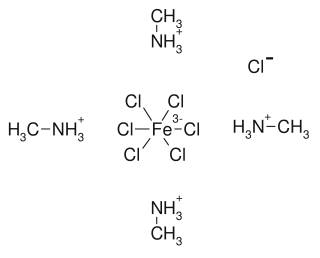
Tetrakis(methylammonium) hexachloroferrate(III) chloride is a chemical compound with the formula (CH3NH3)4[FeCl6]Cl.
The salt extraction process is an electrolytic method which may be used to extract valuable metals from slag, low-grade ores, or other materials by using molten salts. This method was developed by S. Seetharaman, O. Grinder, L. Teng and X. Ge at the Royal Institute of Technology in Sweden as part of a large Steel Eco-Cycle Project in 2005.

Chlorobis(dppe)iron hydride is a coordination complex with the formula HFeCl(dppe)2, where dppe is the bidentate ligand 1,2-bis(diphenylphosphino)ethane. It is a red-violet solid. The compound has attracted much attention as a precursor to dihydrogen complexes.








