
Superconductivity is a phenomenon of exactly zero electrical resistance and expulsion of magnetic flux fields occurring in certain materials, called superconductors, when cooled below a characteristic critical temperature. It was discovered by Dutch physicist Heike Kamerlingh Onnes on April 8, 1911, in Leiden. Like ferromagnetism and atomic spectral lines, superconductivity is a quantum mechanical phenomenon. It is characterized by the Meissner effect, the complete ejection of magnetic field lines from the interior of the superconductor during its transitions into the superconducting state. The occurrence of the Meissner effect indicates that superconductivity cannot be understood simply as the idealization of perfect conductivity in classical physics.

A gyrocompass is a type of non-magnetic compass which is based on a fast-spinning disc and the rotation of the Earth to find geographical direction automatically. The use of a gyrocompass is one of the seven fundamental ways to determine the heading of a vehicle. Although one important component of a gyrocompass is a gyroscope, these are not the same devices; a gyrocompass is built to use the effect of gyroscopic precession, which is a distinctive aspect of the general gyroscopic effect. Gyrocompasses are widely used for navigation on ships, because they have two significant advantages over magnetic compasses:

High-temperature superconductors are materials that behave as superconductors at unusually high temperatures. The first high-Tc superconductor was discovered in 1986 by IBM researchers Georg Bednorz and K. Alex Müller, who were awarded the 1987 Nobel Prize in Physics "for their important break-through in the discovery of superconductivity in ceramic materials".

In physics and materials science, the Curie temperature (TC), or Curie point, is the temperature above which certain materials lose their permanent magnetic properties, to be replaced by induced magnetism. The Curie temperature is named after Pierre Curie, who showed that magnetism was lost at a critical temperature.

Iron(III) oxide or ferric oxide is the inorganic compound with the formula Fe2O3. It is one of the three main oxides of iron, the other two being iron(II) oxide (FeO), which is rare; and iron(II,III) oxide (Fe3O4), which also occurs naturally as the mineral magnetite. As the mineral known as hematite, Fe2O3 is the main source of iron for the steel industry. Fe2O3 is readily attacked by acids. Iron(III) oxide is often called rust, and to some extent this label is useful, because rust shares several properties and has a similar composition. To a chemist, rust is considered an ill-defined material, described as hydrated ferric oxide.
In physics, a ferrimagnetic material is one that has populations of atoms with opposing magnetic moments, as in antiferromagnetism; however, in ferrimagnetic materials, the opposing moments are unequal and a spontaneous magnetization remains. This happens when the populations consist of different materials or ions (such as Fe2+ and Fe3+).
In physics, a ferromagnetic material is said to have magnetocrystalline anisotropy if it takes more energy to magnetize it in certain directions than in others. These directions are usually related to the principal axes of its crystal lattice. It is a special case of magnetic anisotropy.
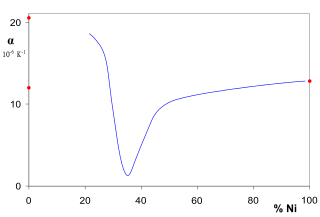
Invar, also known generically as FeNi36, is a nickel–iron alloy notable for its uniquely low coefficient of thermal expansion. The name Invar comes from the word invariable, referring to its relative lack of expansion or contraction with temperature changes.

Iron(II,III) oxide is the chemical compound with formula Fe3O4. It occurs in nature as the mineral magnetite. It is one of a number of iron oxides, the others being iron(II) oxide (FeO), which is rare, and iron(III) oxide (Fe2O3) also known as hematite. It contains both Fe2+ and Fe3+ ions and is sometimes formulated as FeO ∙ Fe2O3. This iron oxide is encountered in the laboratory as a black powder. It exhibits permanent magnetism and is ferrimagnetic, but is sometimes incorrectly described as ferromagnetic. Its most extensive use is as a black pigment. For this purpose, it is synthesised rather than being extracted from the naturally occurring mineral as the particle size and shape can be varied by the method of production.
A samarium–cobalt (SmCo) magnet, a type of rare earth magnet, is a strong permanent magnet made of two basic elements samarium and cobalt. Actually, samarium is substituted by a portion of other rare earth elements including praseodymium, cerium and gadolinium, and cobalt is substituted by a portion of other transition metals including iron, copper and zirconium. They are available in two "series", namely SmCo5 magnets and Sm2Co17 magnets. They were developed in the early 1960s based on work done by Karl Strnat and Alden Ray at Wright-Patterson Air Force Base and the University of Dayton, respectively. In particular, Strnat and Ray developed the first formulation of SmCo5. They are generally ranked similarly in strength to neodymium magnets, but have higher temperature ratings and higher coercivity. They are brittle, and prone to cracking and chipping. Samarium–cobalt magnets have maximum energy products (BHmax) that range from 14 megagauss-oersteds (MG·Oe) to 33 MG·Oe, that is approx. 112 kJ/m3 to 264 kJ/m3; their theoretical limit is 34 MG·Oe, about 272 kJ/m3.
Spontaneous magnetization is the appearance of an ordered spin state (magnetization) at zero applied magnetic field in a ferromagnetic or ferrimagnetic material below a critical point called the Curie temperature or TC.

At atmospheric pressure, three allotropic forms of iron exist: alpha iron (α), gamma iron (γ), and delta iron (δ). At very high pressure, a fourth form exists, called epsilon iron (ε) hexaferrum. Some controversial experimental evidence exists for another high-pressure form that is stable at very high pressures and temperatures.

Manganese(III) oxide is a chemical compound with the formula Mn2O3.
Iron(II) selenide refers to a number of inorganic compounds of ferrous iron and selenide (Se2−). The phase diagram of the system Fe–Se reveals the existence of several non-stoichiometric phases between ~49 at. % Se and ~53 at. % Fe, and temperatures up to ~450 °C. The low temperature stable phases are the tetragonal PbO-structure (P4/nmm) β-Fe1−xSe and α-Fe7Se8. The high temperature phase is the hexagonal, NiAs structure (P63/mmc) δ-Fe1−xSe. Iron (II) selenide occurs naturally as the NiAs-structure mineral achavalite.

An Exchange Spring Magnet is a magnetic material with high coercivity and high saturation properties derived from the exchange interaction between a hard magnetic material and a soft magnetic material, respectively. Coehoorn et al. were the first able to observe an actual exchange spring magnet. Exchange spring magnets are cheaper than many magnets containing rare earth/transition metals, as the hard phase of the magnet can be less than 15% of the overall magnet by volume.

Lithium iridate, Li2IrO3, is a chemical compound of lithium, iridium and oxygen. It forms black crystals with three slightly different layered atomic structures, α, β, and sometimes γ. Lithium iridate exhibits metal-like, temperature-independent electrical conductivity, and changes its magnetic ordering from paramagnetic to antiferromagnetic upon cooling to 15 K.
















