
Tumor necrosis factor is a cell signaling protein (cytokine) involved in systemic inflammation and is one of the cytokines that make up the acute phase reaction. It is produced chiefly by activated macrophages, although it can be produced by many other cell types such as CD4+ lymphocytes, NK cells, neutrophils, mast cells, eosinophils, and neurons. TNFα is a member of the TNF superfamily, consisting of various transmembrane proteins with a homologous TNF domain.
Cytotoxicity is the quality of being toxic to cells. Examples of toxic agents are an immune cell or some types of venom, e.g. from the puff adder or brown recluse spider.
A suicide gene, in genetics, will cause a cell to kill itself through apoptosis. Activation of these genes can be due to many processes, but the main cellular "switch" to induce apoptosis is the p53 protein. Stimulation or introduction of suicide genes is a potential way of treating cancer or other proliferative diseases. Suicide genes form the basis of a strategy for making cancer cells more vulnerable, more sensitive to chemotherapy. The approach has been to attach parts of genes expressed in cancer cells to other genes for enzymes not found in mammals that can convert a harmless substance into one that is toxic to the tumor. Most suicide genes mediate this sensitivity by coding for viral or bacterial enzymes that convert an inactive drug into toxic antimetabolites that inhibit the synthesis of nucleic acid. Suicide genes must be introduced into the cells in ways that ensure their uptake and expression by as many cancer cells as possible, while limiting their expression by normal cells. Suicide gene therapy for cancer requires the vector to have the capacity to discriminate between target and non target cells, between the cancer cells and normal cells.

Pyknosis, or karyopyknosis, is the irreversible condensation of chromatin in the nucleus of a cell undergoing necrosis or apoptosis. It is followed by karyorrhexis, or fragmentation of the nucleus. Pyknosis is also observed in the maturation of erythrocytes and the neutrophil. The maturing metarubricyte will condense its nucleus before expelling it to become a reticulocyte. The maturing neutrophil will condense its nucleus into several connected lobes that stay in the cell until the end of its cell life.

Cell death is the event of a biological cell ceasing to carry out its functions. This may be the result of the natural process of old cells dying and being replaced by new ones, or may result from such factors as disease, localized injury, or the death of the organism of which the cells are part. Apoptosis or Type I cell-death, and autophagy or Type II cell-death are both forms of programmed cell death, while necrosis is a non-physiological process that occurs as a result of infection or injury.
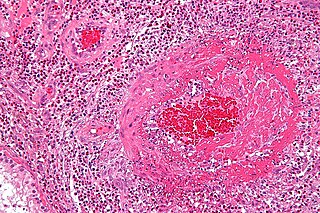
Fibrinoid necrosis is a specific pattern of irreversible, uncontrolled cell death that occurs when antigen-antibody complexes are deposited in the walls of blood vessels along with fibrin. It is common in the immune-mediated vasculitides which are a result of type III hypersensitivity. When stained with hematoxylin and eosin, they appear brightly eosinophilic and smudged.

Avascular necrosis (AVN), also called osteonecrosis or bone infarction, is death of bone tissue due to interruption of the blood supply. Early on, there may be no symptoms. Gradually joint pain may develop which may limit the ability to move. Complications may include collapse of the bone or nearby joint surface.
Acute tubular necrosis (ATN) is a medical condition involving the death of tubular epithelial cells that form the renal tubules of the kidneys. ATN presents with acute kidney injury (AKI) and is one of the most common causes of AKI. Common causes of ATN include low blood pressure and use of nephrotoxic drugs. The presence of "muddy brown casts" of epithelial cells found in the urine during urinalysis is pathognomonic for ATN. Management relies on aggressive treatment of the factors that precipitated ATN. Because the tubular cells continually replace themselves, the overall prognosis for ATN is quite good if the underlying cause is corrected, and recovery is likely within 7 to 21 days.
Coagulative necrosis is a type of accidental cell death typically caused by ischemia or infarction. In coagulative necrosis the architecture of dead tissue is preserved for at least a couple of days. It is believed that the injury denatures structural proteins as well as lysosomal enzymes thus blocking the proteolysis of the damaged cells. The lack of lysosomal enzymes allows it to maintain a "coagulated" morphology for some time. Like most types of necrosis if enough viable cells are present around the affected area regeneration will usually occur.
Liquefactive necrosis is a type of necrosis which results in a transformation of the tissue into a liquid viscous mass. Often it is associated with focal bacterial or fungal infections, and can also manifest as one of the symptoms of an internal chemical burn. In liquefactive necrosis, the affected cell is completely digested by hydrolytic enzymes, resulting in a soft, circumscribed lesion consisting of pus and the fluid remains of necrotic tissue. Dead leukocytes will remain as a creamy yellow pus. After the removal of cell debris by white blood cells, a fluid filled space is left. It is generally associated with abscess formation and is commonly found in the central nervous system.

Caseous necrosis is a form of cell death in which the tissue maintains a cheese-like appearance. The dead tissue appears as a soft and white proteinaceous dead cell mass.

In the field of cell biology, TNF-related apoptosis-inducing ligand (TRAIL), is a protein functioning as a ligand that induces the process of cell death called apoptosis.
Cell damage is a variety of changes of stress that a cell suffers due to external as well internal environmental changes. Among other causes, this can be due to physical, chemical, infectious, biological, nutritional or immunological factors. Cell damage can be reversible or irreversible. Depending on the extent of injury, the cellular response may be adaptive and where possible, homeostasis is restored. Cell death occurs when the severity of the injury exceeds the cell’s ability to repair itself. Cell death is relative to both the length of exposure to a harmful stimulus and the severity of the damage caused. Cell death may occur by necrosis or apoptosis.

The tumor necrosis factor receptor superfamily (TNFRSF) is a protein superfamily of cytokine receptors characterized by the ability to bind tumor necrosis factors (TNFs) via an extracellular cysteine-rich domain. With the exception of nerve growth factor (NGF), all TNFs are homologous to the archetypal TNF-alpha. In their active form, the majority of TNF receptors form trimeric complexes in the plasma membrane. Accordingly, most TNF receptors contain transmembrane domains (TMDs), although some can be cleaved into soluble forms, and some lack a TMD entirely. In addition, most TNF receptors require specific adaptor protein such as TRADD, TRAF, RIP and FADD for downstream signalling. TNF receptors are primarily involved in apoptosis and inflammation, but they can also take part in other signal transduction pathways, such as proliferation, survival, and differentiation. TNF receptors are expressed in a wide variety of tissues in mammals, especially in leukocytes.

Tumor necrosis factor receptor 1 (TNFR1), also known as tumor necrosis factor receptor superfamily member 1A (TNFRSF1A) and CD120a, is a ubiquitous membrane receptor that binds tumor necrosis factor-alpha (TNFα).

A proliferation-inducing ligand (APRIL), also known as tumor necrosis factor ligand superfamily member 13 (TNFSF13), is a protein of the TNF superfamily recognized by the cell surface receptor TACI.

Tumor necrosis factor receptor superfamily member 18 (TNFRSF18) also known as activation-inducible TNFR family receptor (AITR) or glucocorticoid-induced TNFR-related protein (GITR) is a protein that in humans is encoded by the TNFRSF18 gene. GITR is currently of interest to immunologists as a co-stimulatory immune checkpoint molecule.
Ischemic cell death, or oncosis, is a form of accidental cell death. The process is characterized by an ATP depletion within the cell leading to impairment of ionic pumps, cell swelling, clearing of the cytosol, dilation of the Endoplasmic Reticulum and Golgi, mitochondrial condensation, chromatin clumping, and cytoplasmic bleb formation. Oncosis refers to a series of cellular reactions following injury that precedes cell death. The process of oncosis is divided into three stages. First, the cell becomes committed to oncosis as a result of damage incurred to the plasma membrane through toxicity or ischemia, resulting in the leak of ions and water due to ATP depletion. The ionic imbalance that occurs subsequently causes the cell to swell without a concurrent change in membrane permeability to reverse the swelling. Stage two the reversibility threshold for the cell is passed and it becomes committed to cell death. During this stage the membrane becomes abnormally permeable to trypan blue and propidium iodide, indicating membrane compromise. The final stage is cell death and removal of the cell via phagocytosis mediated by an inflammatory response.

Necroptosis is a programmed form of necrosis, or inflammatory cell death. Conventionally, necrosis is associated with unprogrammed cell death resulting from cellular damage or infiltration by pathogens, in contrast to orderly, programmed cell death via apoptosis. The discovery of necroptosis showed that cells can execute necrosis in a programmed fashion and that apoptosis is not always the preferred form of cell death. Furthermore, the immunogenic nature of necroptosis favors its participation in certain circumstances, such as aiding defense pathogens by the immune system. Necroptosis is well defined as a viral defense mechanism, allowing the cell to undergo "cellular suicide" in a caspase-independent fashion in the presence of viral caspase inhibitors to restrict virus replication. In addition to being a response to disease, necroptosis has also been characterized as a component of inflammatory diseases such as Crohn's disease, pancreatitis, and myocardial infarction.














