
In stereochemistry, stereoisomerism, or spatial isomerism, is a form of isomerism in which molecules have the same molecular formula and sequence of bonded atoms (constitution), but differ in the three-dimensional orientations of their atoms in space. This contrasts with structural isomers, which share the same molecular formula, but the bond connections or their order differs. By definition, molecules that are stereoisomers of each other represent the same structural isomer.
Octane is a hydrocarbon and an alkane with the chemical formula C8H18, and the condensed structural formula CH3(CH2)6CH3. Octane has many structural isomers that differ by the amount and location of branching in the carbon chain. One of these isomers, 2,2,4-trimethylpentane (isooctane) is used as one of the standard values in the octane rating scale.

In chemistry, an enantiomer, also known as an optical isomer, is one of two stereoisomers that are mirror images of each other that are non-superposable, much as one's left and right hands have the same shape except for being reversed along one axis. A single chiral atom or similar structural feature in a compound causes that compound to have two possible structures which are non-superposable, each a mirror image of the other. Each member of the pair is termed an enantiomorph ; the structural property is termed enantiomerism. The presence of multiple chiral features in a given compound increases the number of geometric forms possible, though there may still be some perfect-mirror-image pairs.
Diastereomers are a type of a stereoisomer. Diasteoreomers are defined as non-mirror image non-identical stereoisomers. Hence, they occur when two or more stereoisomers of a compound have different configurations at one or more of the equivalent (related) stereocenters and are not mirror images of each other.
When two diastereoisomers differ from each other at only one stereocenter they are epimers. Each stereocenter gives rise to two different configurations and thus typically increases the number of stereoisomers by a factor of two.

A meso compound or meso isomer is a non-optically active member of a set of stereoisomers, at least two of which are optically active. This means that despite containing two or more stereogenic centers, the molecule is not chiral. A meso compound is "superposable" on its mirror image. Two objects can be superposed if all aspects of the objects coincide and it does not produce a "(+)" or "(-)" reading when analyzed with a polarimeter.

Chirality is a geometric property of some molecules and ions. A chiral molecule/ion is non-superposable on its mirror image. The presence of an asymmetric carbon center is one of several structural features that induce chirality in organic and inorganic molecules. The term chirality is derived from the Ancient Greek word for hand, χεῖρ (kheir).

Enantioselective synthesis, also called asymmetric synthesis, is a form of chemical synthesis. It is defined by IUPAC as: a chemical reaction in which one or more new elements of chirality are formed in a substrate molecule and which produces the stereoisomeric products in unequal amounts.
Chiral column chromatography is a variant of column chromatography in which the stationary phase contains a single enantiomer of a chiral compound rather than being achiral. The two enantiomers of the same analyte compound differ in affinity to the single-enantiomer stationary phase and therefore they exit the column at different times.

Borneol is a bicyclic organic compound and a terpene derivative. The hydroxyl group in this compound is placed in an endo position. There are two different enantiomers of borneol. Both d-(+)-borneol and l-(–)-borneol are found in nature.
Chiral resolution in stereochemistry is a process for the separation of racemic compounds into their enantiomers. It is an important tool in the production of optically active drugs. Other terms with the same meaning are optical resolution and mechanical resolution.

Flunoxaprofen, also known as Priaxim, is a chiral nonsteroidal anti-inflammatory drug (NSAID). It is closely related to naproxen, which is also an NSAID. Flunoxaprofen has been shown to significantly improve the symptoms of osteoarthritis and rheumatoid arthritis. The clinical use of flunoxaprofen has ceased due to concerns of potential hepatotoxicity.

Dextrorotation and levorotation are terms used to describe the rotation of plane-polarized light. From the point of view of the observer, dextrorotation refers to clockwise rotation while levorotation refers to counterclockwise rotation.
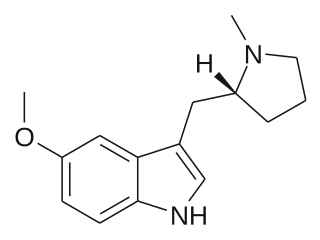
5-MeO-MPMI is a tryptamine derivative that is a psychedelic drug. It was first developed by the team led by JE Macor in 1992, and subsequently investigated by the team led by David Nichols from Purdue University in the late 1990s. This compound produces psychedelic-appropriate responding in animal tests with a similar potency to the amphetamine-derived psychedelic DOI, and has two enantiomers, with only the (R)-enantiomer being active.
In chemistry, isomers are ions or molecules with identical formulas but distinct structures. Isomers do not necessarily share similar properties. Two main forms of isomerism are structural isomerism and stereoisomerism.

3-Methylhexane is a branched hydrocarbon with two enantiomers. It is one of the isomers of heptane.

Chirality is a property of asymmetry important in several branches of science. The word chirality is derived from the Greek χειρ (kheir), "hand," a familiar chiral object.

Pseudophenmetrazine is a psychostimulant compound of the morpholine class. It is the N-demethylated and cis-configured analogue of phendimetrazine as well as the cis-configured stereoisomer of phenmetrazine. In addition, along with phenmetrazine, it is believed to be one of the active metabolites of phendimetrazine, which itself is inactive and behaves merely as a prodrug. Relative to phenmetrazine, pseudophenmetrazine is of fairly low potency, acting as a modest releasing agent of norepinephrine (EC50 = 514 nM), while its (+)-enantiomer is a weak releaser of dopamine (EC50 = 1,457 nM) whereas its (−)-enantiomer is a weak reuptake inhibitor of dopamine (Ki = 2,691 nM); together as a racemic mixture with the two enantiomers combined, pseudophenmetrazine behaves overall more as a dopamine reuptake inhibitor (Ki = 2,630 nM), possibly due to the (+)-enantiomer blocking the uptake of the (−)-enantiomer into dopaminergic neurons and thus preventing it from inducing dopamine release. Neither enantiomer has any significant effect on serotonin reuptake or release (both Ki = >10,000 nM and EC50 = >10,000 nM, respectively).













