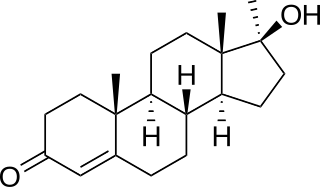
Methyltestosterone, sold under the brand names Android, Metandren, and Testred among others, is an androgen and anabolic steroid (AAS) medication which is used in the treatment of low testosterone levels in men, delayed puberty in boys, at low doses as a component of menopausal hormone therapy for menopausal symptoms like hot flashes, osteoporosis, and low sexual desire in women, and to treat breast cancer in women. It is taken by mouth or held in the cheek or under the tongue.
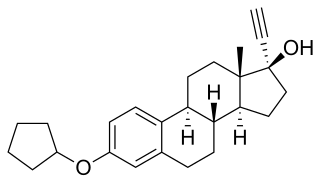
Quinestrol, also known as ethinylestradiol cyclopentyl ether (EECPE), sold under the brand name Estrovis among others, is an estrogen medication which has been used in menopausal hormone therapy, hormonal birth control, and to treat breast cancer and prostate cancer. It is taken once per week to once per month by mouth.

Quinbolone, sold under the brand names Anabolicum and Anabolvis, is an androgen and anabolic steroid (AAS) which was previously marketed in Italy. It was developed by Parke-Davis as a viable orally administered AAS with little or no liver toxicity.
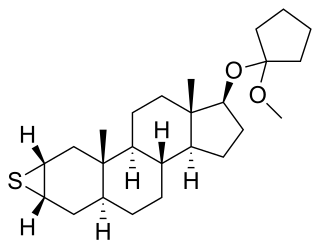
Mepitiostane, sold under the brand name Thioderon, is an orally active antiestrogen and anabolic–androgenic steroid (AAS) of the dihydrotestosterone (DHT) group which is marketed in Japan as an antineoplastic agent for the treatment of breast cancer. It is a prodrug of epitiostanol. The drug was patented and described in 1968.

Methandriol, also known as methylandrostenediol, is an androgen and anabolic steroid (AAS) medication which was developed by Organon and is used in both oral and injectable formulations. It is an orally active 17α-alkylated AAS and a derivative of the endogenous androgen prohormone androstenediol.

Epitiostanol, sold under the brand name Thiodrol, is an injected antiestrogen and anabolic–androgenic steroid (AAS) of the dihydrotestosterone (DHT) group which was described in the literature in 1965 and has been marketed in Japan as an antineoplastic agent for the treatment of breast cancer since 1977.

Estriol succinate, sold under the brand name Synapause among others, is an estrogen medication which is used in the treatment of menopausal symptoms. It is taken by mouth, in through the vagina, and by injection.

Quingestanol, also known as norethisterone 3-cyclopentyl enol ether, is a progestin of the 19-nortestosterone group which was never marketed. It is a prodrug of norethisterone. An acylated derivative, quingestanol acetate, is used as a pharmaceutical drug.
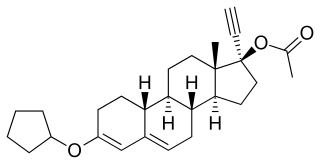
Quingestanol acetate, sold under the brand names Demovis and Pilomin among others, is a progestin medication which was used in birth control pills but is no longer marketed. It is taken by mouth.

Quinestradol, also known as quinestradiol or quinestriol, as well as estriol 3-cyclopentyl ether (E3CPE), is a synthetic estrogen and estrogen ether which is no longer marketed. It is the 3-cyclopentyl ether of estriol. The medication has been studied in the treatment of stress incontinence in elderly women, with effectiveness observed.
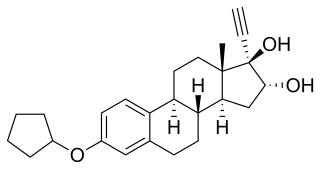
Nilestriol, also known as nylestriol, is a synthetic estrogen which was patented in 1971 and is marketed in China. It is the 3-cyclopentyl ether of ethinylestriol, and is also known as ethinylestriol cyclopentyl ether (EE3CPE). Nilestriol is a prodrug of ethinylestriol, and is a more potent estrogen in comparison. It is described as a slowly-metabolized, long-acting estrogen and derivative of estriol. Nilestriol was assessed in combination with levonorgestrel for the potential treatment of postmenopausal osteoporosis, but this formulation ultimately was not marketed.

Orestrate, also known as estradiol 3-propionate 17β-(1-cyclohexenyl) ether, is an estrogen medication and estrogen ester which was never marketed. It is the C3 propionate ester and C17β-(1-cyclohexenyl) ether of estradiol.

Methylestradiol, sold under the brand names Ginecosid, Ginecoside, Mediol, and Renodiol, is an estrogen medication which is used in the treatment of menopausal symptoms. It is formulated in combination with normethandrone, a progestin and androgen/anabolic steroid medication. Methylestradiol is taken by mouth.

A progestogen ester is an ester of a progestogen or progestin. The prototypical progestogen is progesterone, an endogenous sex hormone. Esterification is frequently employed to improve the pharmacokinetics of steroids, including oral bioavailability, lipophilicity, and elimination half-life. In addition, with intramuscular injection, steroid esters are often absorbed more slowly into the body, allowing for less frequent administration. Many steroid esters function as prodrugs.

Quingestrone, also known as progesterone 3-cyclopentyl enol ether (PCPE) and sold under the brand name Enol-Luteovis, is a progestin medication which was previously used in birth control pills in Italy but is now no longer marketed. It is taken by mouth.

Pentagestrone, also known as 17α-hydroxyprogesterone 3-cyclopentyl enol ether, is a steroidal progestin of the 17α-hydroxyprogesterone group that was never marketed. An acetate ester, pentagestrone acetate, has been marketed for clinical use. Pentagestrone was described in the literature in 1960.

Pentagestrone acetate (PGA), sold under the brand names Gestovis and Gestovister, is a progestin which was described in the literature in 1960 and was introduced by Vister in Italy in 1961. It is the 3-cyclopentyl enol ether of 17α-hydroxyprogesterone acetate. PGA, along with quingestrone, is said to have very similar properties to those of dydrogesterone, a pure progestogen and close analogue of progesterone.

Methyltestosterone 3-hexyl ether, or 17α-methyltestosterone 3-hexyl enol ether, also known as 17α-methylandrost-3,5-dien-17β-ol-3-one 3-hexyl ether, is a synthetic anabolic-androgenic steroid and an androgen ether – specifically, the 3-hexyl ether of methyltestosterone.

Progesterone 3-acetyl enol ether, also known as progesterone acetate, as well as 3-acetoxypregna-3,5-dien-20-one, is a progestin which was never marketed. It was reported to possess similar potency to progesterone and hydroxyprogesterone caproate in the rabbit endometrial carbonic anhydrase test, a bioassay of progestogenic activity. In addition, it was able to maintain pregnancy in animals. Progesterone 3-acetyl enol ether is closely related to quingestrone, which is also known as progesterone 3-cyclopentyl enol ether and was formerly marketed as an oral contraceptive.



















