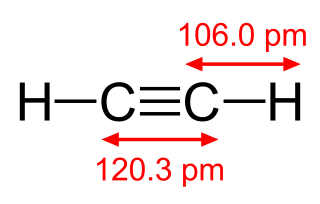
Acetylene is the chemical compound with the formula C2H2 and structure H−C≡C−H. It is a hydrocarbon and the simplest alkyne. This colorless gas is widely used as a fuel and a chemical building block. It is unstable in its pure form and thus is usually handled as a solution. Pure acetylene is odorless, but commercial grades usually have a marked odor due to impurities such as divinyl sulfide and phosphine.

In organic chemistry, an aldehyde is an organic compound containing a functional group with the structure R−CH=O. The functional group itself can be referred to as an aldehyde but can also be classified as a formyl group. Aldehydes are a common motif in many chemicals important in technology and biology.
Acetaldehyde (IUPAC systematic name ethanal) is an organic chemical compound with the formula CH3CHO, sometimes abbreviated as MeCHO. It is a colorless liquid or gas, boiling near room temperature. It is one of the most important aldehydes, occurring widely in nature and being produced on a large scale in industry. Acetaldehyde occurs naturally in coffee, bread, and ripe fruit, and is produced by plants. It is also produced by the partial oxidation of ethanol by the liver enzyme alcohol dehydrogenase and is a contributing cause of hangover after alcohol consumption. Pathways of exposure include air, water, land, or groundwater, as well as drink and smoke. Consumption of disulfiram inhibits acetaldehyde dehydrogenase, the enzyme responsible for the metabolism of acetaldehyde, thereby causing it to build up in the body.
In chemistry, a hydration reaction is a chemical reaction in which a substance combines with water. In organic chemistry, water is added to an unsaturated substrate, which is usually an alkene or an alkyne. This type of reaction is employed industrially to produce ethanol, isopropanol, and butan-2-ol.

Acrylic acid (IUPAC: propenoic acid) is an organic compound with the formula CH2=CHCOOH. It is the simplest unsaturated carboxylic acid, consisting of a vinyl group connected directly to a carboxylic acid terminus. This colorless liquid has a characteristic acrid or tart smell. It is miscible with water, alcohols, ethers, and chloroform. More than a million tons are produced annually.
The Tishchenko reaction is an organic chemical reaction that involves disproportionation of an aldehyde in the presence of an alkoxide. The reaction is named after Russian organic chemist Vyacheslav Tishchenko, who discovered that aluminium alkoxides are effective catalysts for the reaction.
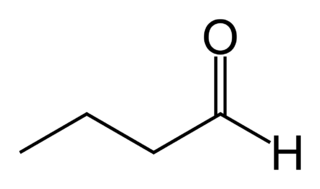
Butyraldehyde, also known as butanal, is an organic compound with the formula CH3(CH2)2CHO. This compound is the aldehyde derivative of butane. It is a colorless flammable liquid with an unpleasant smell. It is miscible with most organic solvents.

Methacrylic acid, abbreviated MAA, is an organic compound with the formula CH2=C(CH3)COOH. This colorless, viscous liquid is a carboxylic acid with an acrid unpleasant odor. It is soluble in warm water and miscible with most organic solvents. Methacrylic acid is produced industrially on a large scale as a precursor to its esters, especially methyl methacrylate (MMA), and to poly(methyl methacrylate) (PMMA).
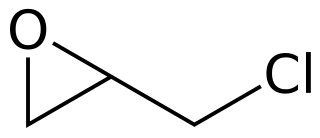
Epichlorohydrin is an organochlorine compound and an epoxide. Despite its name, it is not a halohydrin. It is a colorless liquid with a pungent, garlic-like odor, moderately soluble in water, but miscible with most polar organic solvents. It is a chiral molecule generally existing as a racemic mixture of right-handed and left-handed enantiomers. Epichlorohydrin is a highly reactive electrophilic compound and is used in the production of glycerol, plastics, epoxy glues and resins, epoxy diluents and elastomers.
Synthetic resins are industrially produced resins, typically viscous substances that convert into rigid polymers by the process of curing. In order to undergo curing, resins typically contain reactive end groups, such as acrylates or epoxides. Some synthetic resins have properties similar to natural plant resins, but many do not.

Crotonaldehyde is a chemical compound with the formula CH3CH=CHCHO. The compound is usually sold as a mixture of the E- and Z-isomers, which differ with respect to the relative position of the methyl and formyl groups. The E-isomer is more common (data given in Table is for the E-isomer). This lachrymatory liquid is moderately soluble in water and miscible in organic solvents. As an unsaturated aldehyde, crotonaldehyde is a versatile intermediate in organic synthesis. It occurs in a variety of foodstuffs, e.g. soybean oils.
The Blanc chloromethylation is the chemical reaction of aromatic rings with formaldehyde and hydrogen chloride to form chloromethyl arenes. The reaction is catalyzed by Lewis acids such as zinc chloride. The reaction was discovered by Gustave Louis Blanc (1872-1927) in 1923

Methyl acrylate is an organic compound, more accurately the methyl ester of acrylic acid. It is a colourless liquid with a characteristic acrid odor. It is mainly produced to make acrylate fiber, which is used to weave synthetic carpets. It is also a reagent in the synthesis of various pharmaceutical intermediates. Owing to the tendency of methyl acrylate to polymerize, samples typically contain an inhibitor such as hydroquinone.
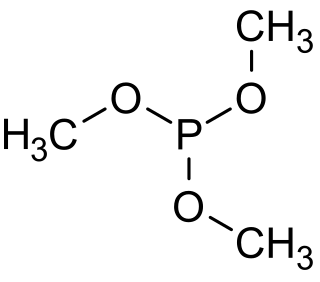
Trimethyl phosphite is an organophosphorus compound with the formula P(OCH3)3, often abbreviated P(OMe)3. It is a colorless liquid with a highly pungent odor. It is the simplest phosphite ester and finds used as a ligand in organometallic chemistry and as a reagent in organic synthesis. The molecule features a pyramidal phosphorus(III) center bound to three methoxy groups.

In organic chemistry, alkanolamines are organic compounds that contain both hydroxyl and amino functional groups on an alkane backbone. Most alkanolamines are colorless.

Diphenyl ether is the organic compound with the formula (C6H5)2O. It is a colorless, low-melting solid. This, the simplest diaryl ether, has a variety of niche applications.
Butyl acrylate is an organic compound with the formula C4H9O2CCH=CH2. A colorless liquid, it is the butyl ester of acrylic acid. It is used commercially on a large scale as a precursor to poly(butyl acrylate). Especially as copolymers, such materials are used in paints, sealants, coatings, adhesives, fuel, textiles, plastics, and caulk.
In industrial chemistry, carboalkoxylation is a process for converting alkenes to esters. This reaction is a form of carbonylation. A closely related reaction is hydrocarboxylation, which employs water in place of alcohols

Pentaerythritol tetraacrylate is an organic compound. It is a tetrafunctional acrylate ester used as a monomer in the manufacture of polymers. As it is a polymerizable acrylate monomer it is nearly always supplied with a polymerisation inhibitor such as MEHQ added.
Hydroxymethylation is a chemical reaction that installs the CH2OH group. The transformation can be implemented in many ways and applies to both industrial and biochemical processes.