The Mesozoa are minuscule, worm-like parasites of marine invertebrates. Generally, these tiny, elusive creatures consist of a somatoderm of ciliated cells surrounding one or more reproductive cells.

Parazoa are a taxon with sub-kingdom category that is located at the base of the phylogenetic tree of the animal kingdom in opposition to the sub-kingdom Eumetazoa; they group together the most primitive forms, characterized by not having proper tissues or that, in any case, these tissues are only partially differentiated. They generally group a single phylum, Porifera, which lack muscles, nerves and internal organs, which in many cases resembles a cell colony rather than a multicellular organism itself. All other animals are eumetazoans, which do have differentiated tissues.

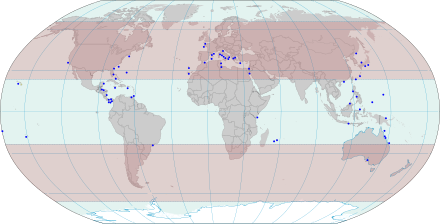
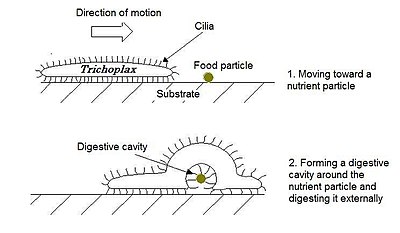
Trichoplax adhaerens is one of the four named species in the phylum Placozoa. The others are Hoilungia hongkongensis, Polyplacotoma mediterranea and Cladtertia collaboinventa. Placozoa is a basal group of multicellular animals, possible relatives of Cnidaria. Trichoplax are very flat organisms commonly less than 4 mm in diameter, lacking any organs or internal structures. They have two cellular layers: the top epitheloid layer is made of ciliated "cover cells" flattened toward the outside of the organism, and the bottom layer is made up of cylinder cells that possess cilia used in locomotion, and gland cells that lack cilia. Between these layers is the fibre syncytium, a liquid-filled cavity strutted open by star-like fibres.
Neuropeptides are chemical messengers made up of small chains of amino acids that are synthesized and released by neurons. Neuropeptides typically bind to G protein-coupled receptors (GPCRs) to modulate neural activity and other tissues like the gut, muscles, and heart.

Coelenterata is a term encompassing the animal phyla Cnidaria and Ctenophora. The name comes from Ancient Greek κοῖλος (koîlos) 'hollow', and ἔντερον (énteron) 'intestine', referring to the hollow body cavity common to these two phyla. They have very simple tissue organization, with only two layers of cells, along with a middle undifferentiated layer called mesoglea, and radial symmetry. Some examples are corals, which are typically colonial; hydrae, jellyfish, sea anemones, and Aurelia, which are solitary; Pennatula; Portuguese man o' war; Gorgonia; and Physalia. Coelenterata lack a specialized circulatory system, relying instead on diffusion across the tissue layers.

Medusozoa is a clade in the phylum Cnidaria, and is often considered a subphylum. It includes the classes Hydrozoa, Scyphozoa, Staurozoa and Cubozoa, and possibly the parasitic Polypodiozoa. Medusozoans are distinguished by having a medusa stage in their often complex life cycle, a medusa typically being an umbrella-shaped body with stinging tentacles around the edge. With the exception of some Hydrozoa, all are called jellyfish in their free-swimming medusa phase.

Animals are multicellular, eukaryotic organisms in the biological kingdom Animalia. With few exceptions, animals consume organic material, breathe oxygen, have myocytes and are able to move, can reproduce sexually, and grow from a hollow sphere of cells, the blastula, during embryonic development. Animals form a clade, meaning that they arose from a single common ancestor.
The Urmetazoan is the hypothetical last common ancestor of all animals, or metazoans. It is universally accepted to be a multicellular heterotroph — with the novelties of a germline and oogamy, an extracellular matrix (ECM) and basement membrane, cell-cell and cell-ECM adhesions and signaling pathways, collagen IV and fibrillar collagen, different cell types, spatial regulation and a complex developmental plan, and relegated unicellular stages.

Planulozoa is a clade which includes the Placozoa, Cnidaria and the Bilateria. The designation Planulozoa may be considered a synonym to Parahoxozoa. Within Planulozoa, the Placozoa may be a sister of Cnidaria to the exclusion of Bilateria. The clade excludes basal animals such as the Ctenophora, and Porifera (sponges). Although this clade was sometimes used to specify a clade of Cnidaria and Bilateria to the exclusion of Placozoa, this is no longer favoured due to recent data indicating a sister group relationship between Cnidaria and Placozoa.

Holozoa is a clade of organisms that includes animals and their closest single-celled relatives, but excludes fungi and all other organisms. Together they amount to more than 1.5 million species of purely heterotrophic organisms, including around 300 unicellular species. It consists of various subgroups, namely Metazoa and the protists Choanoflagellata, Filasterea, Pluriformea and Ichthyosporea. Along with fungi and some other groups, Holozoa is part of the Opisthokonta, a supergroup of eukaryotes. Choanofila was previously used as the name for a group similar in composition to Holozoa, but its usage is discouraged now because it excludes animals and is therefore paraphyletic.

ParaHoxozoa is a clade of animals that consists of Bilateria, Placozoa, and Cnidaria. The relationship of this clade relative to the two other animal lineages Ctenophora and Porifera is debated. Some phylogenomic studies have presented evidence supporting Ctenophora as the sister to Parahoxozoa and Porifera as the sister group to the rest of animals. Other studies have presented evidence supporting Porifera as the sister to Parahoxozoa and Ctenophora as the sister group to the rest of animals, finding that nervous systems either evolved independently in ctenophores and parahoxozoans, or were secondarily lost in poriferans. If ctenophores are taken to have diverged first, Eumetazoa is sometimes used as a synonym for ParaHoxozoa.

Hoilungia is a genus that contains one of the simplest animals and belongs to the phylum Placozoa. Described in 2018, it has only one named species, H. hongkongensis, although there are possible other species. The animal superficially resembles another placozoan, Trichoplax adhaerens, but genetically distinct from it as mitochondrial DNA analysis revealed.
Polyplacotoma mediterranea is a species in the phylum Placozoa, only representative of the genus Polyplacotoma. They differ greatly from other species of placozoans with regards to their morphology and genetic makeup, and have been ranked in the separate class Polyplacotomia. P. mediterranea has the smallest mitogenome, the lowest GC content, and the smallest intergenic spacer regions of all placozoans. Their bodily structure consists of elongated polytomous body branches, as well as a maximum size that is greater than 10 mm in length. The mitochondrial genome of Polyplacotoma mediterranea is also very compact and contains overlapping protein and tRNA gene codes.

Uniplacotomia is a class of placozoans encompassing the vast majority of the phylum, with the exception of Polyplacotoma. It was established in 2022. It comprises the orders Trichoplacea, Cladhexea and Hoilungea. Their morphology is consistent across the class, resembling the typical Trichoplax as mostly rounded, flat organisms rather than the polytomous, branching structure exhibited by Polyplacotomia.

Cladtertia is a genus of placozoan discovered in 2022, whose only currently described species is Cladtertia collaboinventa. However, the genus is known to contain several other species, awaiting a formal description. Its closest described relative is Hoilungia hongkongensis, with whom it forms the order Hoilungea. After Trichoplax, Hoilungia and Polyplacotomia, it is the fourth described placozoan genus up to date.
Hoilungea is a recently created placozoan order comprising Cladtertia, Hoilungia, and other yet-undescribed species. Named in 2022, it is believed to be sister to Cladhexea, and corresponds to Clades III, IV, V and VII of the literature.
Cladhexea is a recently created placozoan order comprising yet-undescribed species. Named in 2022, it is believed to be sister to Hoilungea, and corresponds to Clade VI of the literature.
Polyplacotomia is a class of placozoans, to this date only comprising Polyplacotoma mediterranea. It was established in 2022. Their morphology is strikingly different from other placozoans in Uniplacotomia, exhibiting a highly ramified, branching structure with multiple amoeboid projections. It differs from Uniplacotomia by 76 uniquely present and 600 absent genes.
Hoilungidae is a recently created placozoan family comprising Hoilungia and other yet-undescribed species. Named in 2022, it is believed to be sister to Cladtertiidae, and corresponds to Clades IV, V and VII of the literature.