An aromatic hydrocarbon or arene is a hydrocarbon with sigma bonds and delocalized pi electrons between carbon atoms forming a circle. In contrast, aliphatic hydrocarbons lack this delocalization. The term "aromatic" was assigned before the physical mechanism determining aromaticity was discovered; the term was coined as such simply because many of the compounds have a sweet or pleasant odour. The configuration of six carbon atoms in aromatic compounds is known as a benzene ring, after the simplest possible such hydrocarbon, benzene. Aromatic hydrocarbons can be monocyclic (MAH) or polycyclic (PAH).
Polycyclic aromatic hydrocarbons are hydrocarbons—organic compounds containing only carbon and hydrogen—that are composed of multiple aromatic rings. The simplest such chemicals are naphthalene, having two aromatic rings, and the three-ring compounds anthracene and phenanthrene.
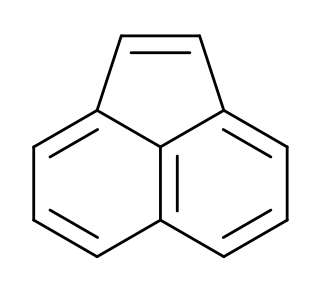
Acenaphthylene is a polycyclic aromatic hydrocarbon. The molecule resembles naphthalene with positions 1 and 8 connected by a C2H2 unit. It is a yellow solid. Unlike many polycyclic aromatic hydrocarbons, it has no fluorescence.
Simple aromatic rings, also known as simple arenes or simple aromatics, are aromatic organic compounds that consist only of a conjugated planar ring system. Many simple aromatic rings have trivial names. They are usually found as substructures of more complex molecules. Typical simple aromatic compounds are benzene, indole, and pyridine.

In chemistry, the organic compound triphenylene is a flat polycyclic aromatic hydrocarbon (PAH) consisting of four fused benzene rings. Triphenylene can be isolated from coal tar. It is also made synthetically by synthesis and trimerization of benzyne. One molecule of triphenylene has delocalized 18-π-electron systems based on a planar structure. It has the molecular formula C
18H
12.

A cyclic compound is a term for a compound in the field of chemistry in which one or more series of atoms in the compound is connected to form a ring. Rings may vary in size from three to many atoms, and include examples where all the atoms are carbon, none of the atoms are carbon, or where both carbon and non-carbon atoms are present. Depending on the ring size, the bond order of the individual links between ring atoms, and their arrangements within the rings, carbocyclic and heterocyclic compounds may be aromatic or non-aromatic, in the latter case, they may vary from being fully saturated to having varying numbers of multiple bonds between the ring atoms. Because of the tremendous diversity allowed, in combination, by the valences of common atoms and their ability to form rings, the number of possible cyclic structures, even of small size numbers in the many billions.

Ovalene is a polycyclic aromatic hydrocarbon with the formula C32H14, which consists of ten peri-fused six-membered rings. It is very similar to coronene.

Chrysene is a polycyclic aromatic hydrocarbon (PAH) with the molecular formula C
18H
12 that consists of four fused benzene rings. It is a natural constituent of coal tar, from which it was first isolated and characterized. It is also found in creosote at levels of 0.5-6 mg/kg.

1H-Phenalene, often called simply phenalene is a polycyclic aromatic hydrocarbon (PAH). Like many PAHs, it is an atmospheric pollutant formed during the combustion of fossil fuels. It is the parent compound for the phosphorus-containing phosphaphenalenes.
Chlorinated polycyclic aromatic hydrocarbons (Cl-PAHs) are a group of compounds comprising polycyclic aromatic hydrocarbons with two or more aromatic rings and one or more chlorine atoms attached to the ring system. Cl-PAHs can be divided into two groups: chloro-substituted PAHs, which have one or more hydrogen atoms substituted by a chlorine atom, and chloro-added Cl-PAHs, which have two or more chlorine atoms added to the molecule. They are products of incomplete combustion of organic materials. They have many congeners, and the occurrences and toxicities of the congeners differ. Cl-PAHs are hydrophobic compounds and their persistence within ecosystems is due to their low water solubility. They are structurally similar to other halogenated hydrocarbons such as polychlorinated dibenzo-p-dioxins (PCDDs), dibenzofurans (PCDFs), and polychlorinated biphenyls (PCBs). Cl-PAHs in the environment are strongly susceptible to the effects of gas/particle partitioning, seasonal sources, and climatic conditions.

Benz[a]anthracene or benzo[a]anthracene is a polycyclic aromatic hydrocarbon with the chemical formula C18H12.

Benzo[k]fluoranthene is an organic compound with the chemical formula C20H12. Classified as a polycyclic aromatic hydrocarbon (PAH), it is a colourless solid that is poorly soluble in most solvents. Impure samples can appear off white. Closely related isomeric compounds include benzo(a)fluoranthene, benzo(b)fluoranthene, benzo(e)fluoranthene, and benzo(j)fluoranthene.
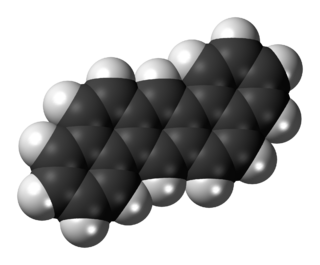
Dibenz[a,h]anthracene is an organic compound with the chemical formula C22H14. It is a polycyclic aromatic hydrocarbon made of five fused benzene rings.
It is a fused five ringed, cyclopenta, PAHs compound which is common as a pollutant of smoke and oils. It is white to light yellow crystalline solid. It is stable and highly genotoxic in bacterial and mammalian cell systems, as it intercalates into DNA and causes mutations.
The unidentified infrared emission bands are infrared discrete emissions from circumstellar regions, interstellar media, star-forming regions and extragalactic objects for which the identity of the emitting materials is unknown. The main infrared features occur around 3.3, 6.2, 7.7, 8.6, 11.2, and 12.7 μm, although there are many other weak emission features within the ~ 5–18 μm spectral range. In the 1980s, astronomers discovered that the origin of the UIR emission bands is inherent in compounds made of aromatic C–H and C=C chemical bonds, and hypothesized that the materials responsible should be polycyclic aromatic hydrocarbon (PAH) molecules. Nevertheless, data recorded with the ESA's Infrared Space Observatory and NASA's Spitzer Space Telescope have suggested that the UIR emission bands arise from compounds that are far more complex in composition and structure than PAH molecules. Moreover, the UIR bands follow a clear evolutionary spectral trend that is linked to the lifespan of the astronomical source; from the time the UIR bands first appear around evolved stars in the protoplanetary nebula stage to evolved stages such as the planetary nebula phase.
Gallaecimonas is a recently described genus of bacteria. The first described species of this genus was Gallaecimonas pentaromativorans gen. nov., sp. nov. isolated by Rodríguez Blanco et al. in 2010 from intertidal sediments of the ria of Corcubión. It is a Gram-negative, rod-shaped, halotolerant bacterium in the class Gammaproteobacteria. It can degrade high molecular mass polycyclic aromatic hydrocarbons of 4 and 5 rings. The 16S rRNA gene sequences of the type strain CEE_131(T) proved to be distantly related to those of Rheinheimera and Serratia. Its G+C content was 41.7 mol%.

Benz[e]acephenanthrylene is an organic compound with the chemical formula C20H12. It is a polycyclic aromatic hydrocarbon (PAH) made of four benzene rings around a 5-membered ring.

Dibenzopyrenes are a group of high molecular weight polycyclic aromatic hydrocarbons with the molecular formula C24H14. There are five isomers of dibenzopyrene which differ by the arrangement of aromatic rings: dibenzo[a,e]pyrene, dibenzo[a,h]pyrene, dibenzo[a,i]pyrene, dibenzo[a,l]pyrene, and dibenzo[e,l]pyrene.
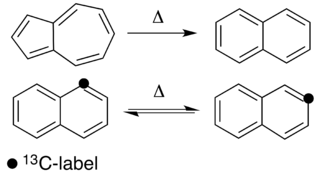
Thermal rearrangements of aromatic hydrocarbons are considered to be unimolecular reactions that directly involve the atoms of an aromatic ring structure and require no other reagent than heat. These reactions can be categorized in two major types: one that involves a complete and permanent skeletal reorganization (isomerization), and one in which the atoms are scrambled but no net change in the aromatic ring occurs (automerization). The general reaction schemes of the two types are illustrated in Figure 1.

Bicalicene is polycyclic hydrocarbon with chemical formula C16H8, composed of linked two cyclopentadiene ring and two cyclopropene ring.














