
Chrome plating, often referred to simply as chrome, is a technique of electroplating a thin layer of chromium onto a metal object. The chromed layer can be decorative, provide corrosion resistance, ease cleaning procedures, or increase surface hardness. Sometimes, a less expensive imitator of chrome may be used for aesthetic purposes.
Plating is a surface covering in which a metal is deposited on a conductive surface. Plating has been done for hundreds of years; it is also critical for modern technology. Plating is used to decorate objects, for corrosion inhibition, to improve solderability, to harden, to improve wearability, to reduce friction, to improve paint adhesion, to alter conductivity, to improve IR reflectivity, for radiation shielding, and for other purposes. Jewelry typically uses plating to give a silver or gold finish.

Diamond-like carbon (DLC) is a class of amorphous carbon material that displays some of the typical properties of diamond. DLC is usually applied as coatings to other materials that could benefit from some of those properties.

Titanium nitride is an extremely hard ceramic material, often used as a coating on titanium alloys, steel, carbide, and aluminium components to improve the substrate's surface properties.
A coating is a covering that is applied to the surface of an object, usually referred to as the substrate. The purpose of applying the coating may be decorative, functional, or both. The coating itself may be an all-over coating, completely covering the substrate, or it may only cover parts of the substrate. An example of all of these types of coating is a product label on many drinks bottles- one side has an all-over functional coating and the other side has one or more decorative coatings in an appropriate pattern to form the words and images.

A superalloy, or high-performance alloy, is an alloy that exhibits several key characteristics: excellent mechanical strength, resistance to thermal creep deformation, good surface stability, and resistance to corrosion or oxidation. The crystal structure is typically face-centered cubic austenitic. Examples of such alloys are Hastelloy, Inconel, Waspaloy, Rene alloys, Incoloy, MP98T, TMS alloys, and CMSX single crystal alloys.

Chromium carbide is a ceramic compound that exists in several different chemical compositions: Cr3C2, Cr7C3,and Cr23C6. At standard conditions it exists as a gray solid. It is extremely hard and corrosion resistant. It is also a refractory compound, which means that it retains its strength at high temperatures as well. These properties make it useful as an additive to metal alloys. When chromium carbide crystals are integrated into the surface of a metal it improves the wear resistance and corrosion resistance of the metal, and maintains these properties at elevated temperatures. The hardest and most commonly used composition for this purpose is Cr3C2.

Nitriding is a heat treating process that diffuses nitrogen into the surface of a metal to create a case-hardened surface. These processes are most commonly used on low-carbon, low-alloy steels. They are also used on medium and high-carbon steels, titanium, aluminium and molybdenum. In 2015, nitriding was used to generate unique duplex microstructure, known to be associated with strongly enhanced mechanical properties
Electroless nickel plating (EN) is an auto-catalytic reaction that deposits an even layer of nickel-phosphorus or nickel-boron alloy on the surface of a solid material, or substrate, like metal or plastic. The process involves dipping the substrate in a bath of plating solution, where a reducing agent, like hydrated sodium hypophosphite (NaPO2H2 · H2O), reacts with the material's ions to deposit the nickel alloy. The metallurgical properties of the alloy depend on the percentage of phosphorus, which can range from 2–5% (low phosphorus) to 11–14% (high phosphorus). Unlike electroplating, it is not necessary to pass an electric current through the plating solution to form a deposit. Electroless plating prevents corrosion and wear, and can be used to manufacture composite coatings by suspending powder in the bath. EN plating creates an even layer regardless of the geometry of the surface – in contrast to electroplating which suffers from flux-density issues as an electromagnetic field will vary due to the surface profile and result in uneven depositions. Depending on the catalyst, EN plating can be applied to non-conductive surfaces.
Electron-beam physical vapor deposition, or EBPVD, is a form of physical vapor deposition in which a target anode is bombarded with an electron beam given off by a charged tungsten filament under high vacuum. The electron beam causes atoms from the target to transform into the gaseous phase. These atoms then precipitate into solid form, coating everything in the vacuum chamber with a thin layer of the anode material.
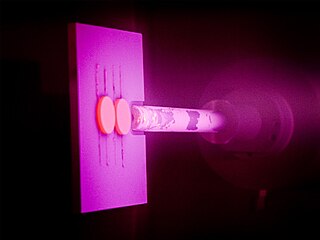
Physical vapor deposition (PVD) describes a variety of vacuum deposition methods which can be used to produce thin films and coatings. PVD is characterized by a process in which the material goes from a condensed phase to a vapor phase and then back to a thin film condensed phase. The most common PVD processes are sputtering and evaporation. PVD is used in the manufacture of items which require thin films for mechanical, optical, chemical or electronic functions. Examples include semiconductor devices such as thin film solar panels, aluminized PET film for food packaging and balloons, and titanium nitride coated cutting tools for metalworking. Besides PVD tools for fabrication, special smaller tools have been developed.

Thermal spraying techniques are coating processes in which melted materials are sprayed onto a surface. The "feedstock" is heated by electrical or chemical means.
Pyrolytic coating is a thin film coating applied at high temperatures and sprayed onto the glass surface during the float glass process.

Gas dynamic cold spraying or cold spraying (CS) is a coating deposition method. Solid powders are accelerated in a supersonic gas jet to velocities up to ca. 1200 m/s. During impact with the substrate, particles undergo plastic deformation and adhere to the surface. To achieve a uniform thickness the spraying nozzle is scanned along the substrate. Metals, polymers, ceramics, composite materials and nanocrystalline powders can be deposited using cold spraying. The kinetic energy of the particles, supplied by the expansion of the gas, is converted to plastic deformation energy during bonding. Unlike thermal spraying techniques, e.g., plasma spraying, arc spraying, flame spraying, or high velocity oxygen fuel (HVOF), the powders are not melted during the spraying process.
Dry lubricants or solid lubricants are materials that, despite being in the solid phase, are able to reduce friction between two surfaces sliding against each other without the need for a liquid oil medium.

Ceramic matrix composites (CMCs) are a subgroup of composite materials as well as a subgroup of ceramics. They consist of ceramic fibres embedded in a ceramic matrix. The matrix and fibres can consist of any ceramic material, whereby carbon and carbon fibres can also be considered a ceramic material.
Combustion chemical vapor deposition (CCVD) is a chemical process by which thin-film coatings are deposited onto substrates in the open atmosphere.
Zinc flake coatings are non-electrolytically applied coatings, which provide good protection against corrosion. These coatings consist of a mixture of zinc and aluminium flakes, which are bonded together by an inorganic matrix.
Nanolamination is the production of materials that are fully dense, ultra-fine grained solids that exhibit a high concentration of interface defects. The properties of fabricated nanolaminates depend on their compositions and thicknesses.












