
Molecular diffusion, often simply called diffusion, is the thermal motion of all particles at temperatures above absolute zero. The rate of this movement is a function of temperature, viscosity of the fluid and the size (mass) of the particles. Diffusion explains the net flux of molecules from a region of higher concentration to one of lower concentration. Once the concentrations are equal the molecules continue to move, but since there is no concentration gradient the process of molecular diffusion has ceased and is instead governed by the process of self-diffusion, originating from the random motion of the molecules. The result of diffusion is a gradual mixing of material such that the distribution of molecules is uniform. Since the molecules are still in motion, but an equilibrium has been established, the end result of molecular diffusion is called a "dynamic equilibrium". In a phase with uniform temperature, absent external net forces acting on the particles, the diffusion process will eventually result in complete mixing.

Fick's laws of diffusion describe diffusion and were derived by Adolf Fick in 1855. They can be used to solve for the diffusion coefficient, D. Fick's first law can be used to derive his second law which in turn is identical to the diffusion equation.

Facilitated diffusion is the process of spontaneous passive transport of molecules or ions across a biological membrane via specific transmembrane integral proteins. Being passive, facilitated transport does not directly require chemical energy from ATP hydrolysis in the transport step itself; rather, molecules and ions move down their concentration gradient reflecting its diffusive nature.

Brine is a high-concentration solution of salt in water. In different contexts, brine may refer to salt solutions ranging from about 3.5% up to about 26%. Lower levels of concentration are called by different names: fresh water, brackish water, and saline water.
Gas exchange is the physical process by which gases move passively by diffusion across a surface. For example, this surface might be the air/water interface of a water body, the surface of a gas bubble in a liquid, a gas-permeable membrane, or a biological membrane that forms the boundary between an organism and its extracellular environment.
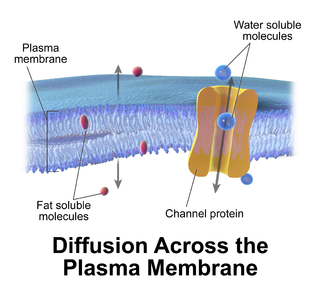
Passive transport is a movement of ions and other atomic or molecular substances across cell membranes without need of energy input. Unlike active transport, it does not require an input of cellular energy because it is instead driven by the tendency of the system to grow in entropy. The rate of passive transport depends on the permeability of the cell membrane, which, in turn, depends on the organization and characteristics of the membrane lipids and proteins. The four main kinds of passive transport are simple diffusion, facilitated diffusion, filtration, and/or osmosis.
A membrane transport protein is a membrane protein involved in the movement of ions, small molecules, or macromolecules, such as another protein, across a biological membrane. Transport proteins are integral transmembrane proteins; that is they exist permanently within and span the membrane across which they transport substances. The proteins may assist in the movement of substances by facilitated diffusion or active transport. The two main types of proteins involved in such transport are broadly categorized as either channels or carriers. The solute carriers and atypical SLCs are secondary active or facilitative transporters in humans.
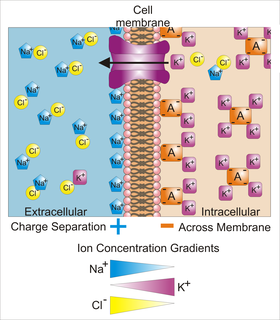
Membrane potential is the difference in electric potential between the interior and the exterior of a biological cell. With respect to the exterior of the cell, typical values of membrane potential, normally given in millivolts, range from –40 mV to –80 mV.
In semiconductor production, doping is the intentional introduction of impurities into an intrinsic semiconductor for the purpose of modulating its electrical, optical and structural properties. The doped material is referred to as an extrinsic semiconductor. A semiconductor doped to such high levels that it acts more like a conductor than a semiconductor is referred to as a degenerate semiconductor.

Cytolysis, or osmotic lysis, occurs when a cell bursts due to an osmotic imbalance that has caused excess water to diffuse into the cell. Water can enter the cell by diffusion through the cell membrane or through selective membrane channels called aquaporins, which greatly facilitate the flow of water. It occurs in a hypotonic environment, where water moves into the cell by osmosis and causes its volume to increase to the point where the volume exceeds the membrane's capacity and the cell bursts. The presence of a cell wall prevents the membrane from bursting, so cytolysis only occurs in animal and protozoa cells which do not have cell walls. The reverse process is plasmolysis.

Crystallization is the process by which a solid forms, where the atoms or molecules are highly organized into a structure known as a crystal. Some of the ways by which crystals form are precipitating from a solution, freezing, or more rarely deposition directly from a gas. Attributes of the resulting crystal depend largely on factors such as temperature, air pressure, and in the case of liquid crystals, time of fluid evaporation.
In semiconductor physics, the depletion region, also called depletion layer, depletion zone, junction region, space charge region or space charge layer, is an insulating region within a conductive, doped semiconductor material where the mobile charge carriers have been diffused away, or have been forced away by an electric field. The only elements left in the depletion region are ionized donor or acceptor impurities.
Vacancy diffusion is a diffusion process whereby the random thermally-activated movement of atoms in a solid results in the net transport of atoms. For example, helium atoms inside a balloon can diffuse through the wall of the balloon and escape, resulting in the balloon slowly deflating. Other air molecules have lower mobilities and thus diffuse more slowly through the balloon wall. There is a concentration gradient in the balloon wall, because the balloon was initially filled with helium, and thus there is plenty of helium on the inside, but there is relatively little helium on the outside. The rate of transport is governed by the diffusivity and the concentration gradient.
In electrochemistry, overpotential is the potential difference (voltage) between a half-reaction's thermodynamically determined reduction potential and the potential at which the redox event is experimentally observed. The term is directly related to a cell's voltage efficiency. In an electrolytic cell the existence of overpotential implies the cell requires more energy than thermodynamically expected to drive a reaction. In a galvanic cell the existence of overpotential means less energy is recovered than thermodynamics predicts. In each case the extra/missing energy is lost as heat. The quantity of overpotential is specific to each cell design and varies across cells and operational conditions, even for the same reaction. Overpotential is experimentally determined by measuring the potential at which a given current density is achieved.

Osmosis is the spontaneous net movement of solvent molecules through a selectively permeable membrane into a region of higher solute concentration, in the direction that tends to equalize the solute concentrations on the two sides. It may also be used to describe a physical process in which any solvent moves across a selectively permeable membrane separating two solutions of different concentrations. Osmosis can be made to do work. Osmotic pressure is defined as the external pressure required to be applied so that there is no net movement of solvent across the membrane. Osmotic pressure is a colligative property, meaning that the osmotic pressure depends on the molar concentration of the solute but not on its identity.
Diffusion Current is a current in a semiconductor caused by the diffusion of charge carriers. This is the current which is due to the transport of charges occurring because of non-uniform concentration of charged particles in a semiconductor. The drift current, by contrast, is due to the motion of charge carriers due to the force exerted on them by an electric field. Diffusion current can be in the same or opposite direction of a drift current. The diffusion current and drift current together are described by the drift–diffusion equation.
The absorption of water by plants is essential for various metabolic activities. Terrestrial plants get their water supply from soil which serves as the source of water and [minerals]. The way in which water from soil enters roots, particularly to the root xylem, is called "mechanism of water absorption". Both active and passive absorption have been proposed for mechanism of water absorption.This process is known as Conduction
Membrane technology covers all engineering approaches for the transport of substances between two fractions with the help of permeable membranes. In general, mechanical separation processes for separating gaseous or liquid streams use membrane technology.
Equimolar counterdiffusion is an instance of molecular diffusion in a binary mixture, and occurs when equal numbers of molecules of the two substances are moving in opposite directions.










