
Nitrate is a polyatomic ion with the molecular formula NO−
3 and a molecular mass of 62.0049 u. Organic compounds that contain the nitrate ester as a functional group (RONO2) are also called nitrates.

In chemistry, a salt is an ionic compound that can be formed by the neutralization reaction of an acid and a base. Salts are composed of related numbers of cations and anions so that the product is electrically neutral. These component ions can be inorganic, such as chloride (Cl−), or organic, such as acetate ; and can be monatomic, such as fluoride (F−), or polyatomic, such as sulfate.

Potassium chloride (KCl) is a metal halide salt composed of potassium and chlorine. It is odorless and has a white or colorless vitreous crystal appearance. The solid dissolves readily in water and its solutions have a salt-like taste. KCl is used as a fertilizer, in medicine, in scientific applications, and in food processing, where it may be known as E number additive E508.
A halide is a binary phase, of which one part is a halogen atom and the other part is an element or radical that is less electronegative than the halogen, to make a fluoride, chloride, bromide, iodide, astatide, or theoretically tennesside compound. The alkali metals combine directly with halogens under appropriate conditions forming halides of the general formula, MX. Many salts are halides; the hal- syllable in halide and halite reflects this correlation. All Group 1 metals form halides that are white solids at room temperature.

Niter, or nitre (chiefly British), is the mineral form of potassium nitrate, KNO3, also known as saltpeter or saltpetre. Historically, the term niter was not well differentiated from natron, both of which have been very vaguely defined but generally refer to compounds of sodium or potassium joined with carbonate or nitrate ions.

Alunite is a hydrated aluminium potassium sulfate mineral, formula KAl3(SO4)2(OH)6. It was first observed in the 15th century at Tolfa, near Rome, where it is mined for the manufacture of alum. First called aluminilite by J.C. Delamétherie in 1797, this name was contracted by François Beudant three decades later to alunite.

Caliche is a sedimentary rock, a hardened natural cement of calcium carbonate that binds other materials—such as gravel, sand, clay, and silt. It occurs worldwide, in aridisol and mollisol soil orders—generally in arid or semiarid regions, including in central and western Australia, in the Kalahari Desert, in the High Plains of the western USA, in the Sonoran Desert and Mojave Desert, and in Eastern Saudi Arabia Al-Hasa. Caliche is also known as calcrete or kankar. It belongs to the duricrusts. The term caliche is Spanish and is originally from the Latin calx, meaning lime.
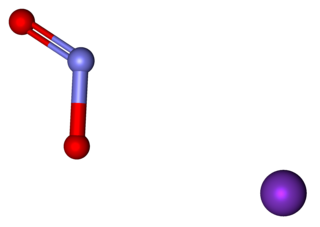
Potassium nitrite (distinct from potassium nitrate) is the inorganic compound with the chemical formula KNO2. It is an ionic salt of potassium ions K+ and nitrite ions NO2−, which forms a white or slightly yellow, hygroscopic crystalline powder that is soluble in water.

An iodate is a conjugate base of iodic acid. In the iodate anion, iodine is bonded to three oxygen atoms and the molecular formula is IO−
3. The molecular geometry of iodate is trigonal pyramidal.

The origins of meat preservation are lost to the ages but probably began when humans began to realize the preservative value of salt. Sausage making originally developed as a means to preserve and transport meat. Primitive societies learned that dried berries and spices could be added to dried meat. The procedure of stuffing meat into casings remains basically the same today, but sausage recipes have been greatly refined and sausage making has become a highly respected culinary art.

Potassium chromate is the inorganic compound with the formula (K2CrO4). This yellow solid is the potassium salt of the chromate anion. It is a common laboratory chemical, whereas sodium chromate is important industrially. It is a class two carcinogen as it contains hexavalent chromium.

Curing salt is used in meat processing to generate a pinkish shade and to extend shelf life. It is both a color agent and a means to facilitate food preservation as it prevents or slows spoilage by bacteria or fungus. Curing salts are generally a mixture of table salt and sodium nitrite and are used for pickling meats as part of the process to make sausage or cured meat such as ham, bacon, pastrami, corned beef, etc. According to the meat industry, the reason for using nitrite-curing salt is not about giving an appetizing red or pink color but to inhibit the growth of bacteria, specifically Clostridium botulinum in an effort to prevent botulism.
Although, in modern usage, the word “nitre” usually refers to the mineral form of potassium nitrate, it may also refer to a variety of other minerals and chemical compounds, including
This page is based on this
Wikipedia article Text is available under the
CC BY-SA 4.0 license; additional terms may apply.
Images, videos and audio are available under their respective licenses.













