
Sulfur dioxide or sulphur dioxide is the chemical compound with the formula SO
2. It is a toxic gas responsible for the odor of burnt matches. It is released naturally by volcanic activity and is produced as a by-product of copper extraction and the burning of sulfur-bearing fossil fuels.

The sulfate or sulphate ion is a polyatomic anion with the empirical formula SO2−4. Salts, acid derivatives, and peroxides of sulfate are widely used in industry. Sulfates occur widely in everyday life. Sulfates are salts of sulfuric acid and many are prepared from that acid.

Sulfites or sulphites are compounds that contain the sulfite ion, SO2−
3. The sulfite ion is the conjugate base of bisulfite. Although its acid is elusive, its salts are widely used.

In chemistry, neutralization or neutralisation is a chemical reaction in which acid and a base react with an equivalent quantity of each other. In a reaction in water, neutralization results in there being no excess of hydrogen or hydroxide ions present in the solution. The pH of the neutralized solution depends on the acid strength of the reactants.

Sodium sulfite (sodium sulphite) is the inorganic compound with the chemical formula Na2SO3. A white, water-soluble solid, it is used commercially as an antioxidant and preservative. A heptahydrate is also known but it is less useful because of its greater susceptibility toward oxidation by air.

In organic chemistry, sulfonic acid refers to a member of the class of organosulfur compounds with the general formula R−S(=O)2−OH, where R is an organic alkyl or aryl group and the S(=O)2(OH) group a sulfonyl hydroxide. As a substituent, it is known as a sulfo group. A sulfonic acid can be thought of as sulfuric acid with one hydroxyl group replaced by an organic substituent. The parent compound is the parent sulfonic acid, HS(=O)2(OH), a tautomer of sulfurous acid, S(=O)(OH)2. Salts or esters of sulfonic acids are called sulfonates.
In chemistry, disproportionation, sometimes called dismutation, is a redox reaction in which one compound of intermediate oxidation state converts to two compounds, one of higher and one of lower oxidation states. More generally, the term can be applied to any desymmetrizing reaction of the following type, regardless of whether it is a redox or some other type of process:

Sodium dithionite is a white crystalline powder with a sulfurous odor. Although it is stable in dry air, it decomposes in hot water and in acid solutions.
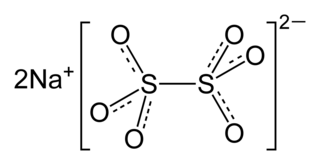
Sodium dithionate Na2S2O6 is an important compound for inorganic chemistry. It is also known under names disodium dithionate, sodium hyposulfate, and sodium metabisulfate. The sulfur can be considered to be in its +5 oxidation state.

The bisulfite ion (IUPAC-recommended nomenclature: hydrogensulfite) is the ion HSO−
3. Salts containing the HSO−
3 ion are also known as "sulfite lyes". Sodium bisulfite is used interchangeably with sodium metabisulfite (Na2S2O5). Sodium metabisulfite dissolves in water to give a solution of Na+HSO−
3.

The dithionite is the oxyanion with the formula [S2O4]2−. It is commonly encountered as the salt sodium dithionite. For historical reasons, it is sometimes called hydrosulfite, but it contains no hydrogen and is not a sulfite. The dianion has a steric number of 4 and trigonal pyramidal geometry.

Potassium bisulfite (or potassium hydrogen sulfite) is a chemical mixture with the approximate chemical formula KHSO3. Potassium bisulfite in fact is not a real compound, but a mixture of salts that dissolve in water to give solutions composed of potassium ions and bisulfite ions. It is a white solid with an odor of sulfur dioxide. Attempts to crystallize potassium bisulfite yield potassium metabisulfite, K2S2O5.
The sulfite process produces wood pulp that is almost pure cellulose fibers by treating wood chips with solutions of sulfite and bisulfite ions. These chemicals cleave the bonds between the cellulose and lignin components of the lignocellulose. A variety of sulfite/bisulfite salts are used, including sodium (Na+), calcium (Ca2+), potassium (K+), magnesium (Mg2+), and ammonium (NH4+). The lignin is converted to lignosulfonates, which are soluble and can be separated from the cellulose fibers. For the production of cellulose, the sulfite process competes with the Kraft process which produces stronger fibers and is less environmentally costly.

Calcium sulfite, or calcium sulphite, is a chemical compound, the calcium salt of sulfite with the formula CaSO3·x(H2O). Two crystalline forms are known, the hemihydrate and the tetrahydrate, respectively CaSO3·½(H2O) and CaSO3·4(H2O). All forms are white solids. It is most notable as the product of flue-gas desulfurization.
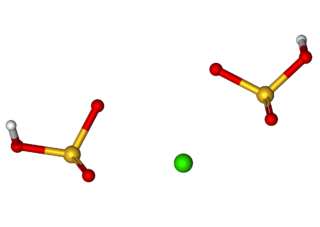
Calcium bisulfite is an inorganic compound which is the salt of a calcium cation and a bisulfite anion. It may be prepared by treating lime with an excess of sulfur dioxide and water. As a food additive it is used as a preservative under the E number E227. Calcium bisulfite is an acid salt and behaves like an acid in aqueous solution. It is used in the sulfite process for producing paper from wood chips.
The Wellman–Lord process is a regenerable process to remove sulfur dioxide from flue gas without creating a throwaway sludge product.

A disulfite, commonly known as metabisulfite or pyrosulfite, is a chemical compound containing the ion S
2O2−
5. It is a colorless dianion that is primarily marketed in the form of sodium metabisulfite or potassium metabisulfite. When dissolved in water, these salts release the hydrogensulfite HSO−
3 anion. These salts act equivalently to sodium hydrogensulfite or potassium hydrogensulfite.

Sodium bisulfite (or sodium bisulphite, sodium hydrogen sulfite) is a chemical mixture with the approximate chemical formula NaHSO3. Sodium bisulfite in fact is not a real compound, but a mixture of salts that dissolve in water to give solutions composed of sodium and bisulfite ions. It appears in form of white or yellowish-white crystals with an odor of sulfur dioxide. For properties of sodium bisulfite, refer to the table located to the right. Regardless of its ill-defined nature, sodium bisulfite is used in many different industries such a food additive with E number E222 in the food industry, a reducing agent in the cosmetic industry, and a decomposer of residual hypochlorite used in the bleaching industry.

Sulfoxylic acid (H2SO2) (also known as hyposulfurous acid or sulfur dihydroxide) is an unstable oxoacid of sulfur in an intermediate oxidation state between hydrogen sulfide and dithionous acid. It consists of two hydroxy groups attached to a sulfur atom. Sulfoxylic acid contains sulfur in an oxidation state of +2. Sulfur monoxide (SO) can be considered as a theoretical anhydride for sulfoxylic acid, but it is not actually known to react with water.
The topic of sulfite food and beverage additives covers the application of sulfites in food chemistry. "Sulfite" is jargon that encompasses a variety of materials that are commonly used as preservatives or food additive in the production of diverse foods and beverages. Although sulfite salts are relatively nontoxic, their use has led to controversy, resulting in extensive regulations. Sulfites are a source of sulfur dioxide (SO2), a bactericide.



















