This page is based on this
Wikipedia article Text is available under the
CC BY-SA 4.0 license; additional terms may apply.
Images, videos and audio are available under their respective licenses.

Aqua regia is a mixture of nitric acid and hydrochloric acid, optimally in a molar ratio of 1:3. Aqua regia is a yellow-orange fuming liquid, so named by alchemists because it can dissolve the noble metals gold and platinum, though not all metals.

In chemistry, water of crystallization or water of hydration are water molecules that are present inside crystals. Water is often incorporated in the formation of crystals from aqueous solutions. In some contexts, water of crystallization is the total mass of water in a substance at a given temperature and is mostly present in a definite (stoichiometric) ratio. Classically, "water of crystallization" refers to water that is found in the crystalline framework of a metal complex or a salt, which is not directly bonded to the metal cation.

Organotin compounds or stannanes are chemical compounds based on tin with hydrocarbon substituents. Organotin chemistry is part of the wider field of organometallic chemistry. The first organotin compound was diethyltin diiodide ((C2H5)2SnI2), discovered by Edward Frankland in 1849. The area grew rapidly in the 1900s, especially after the discovery of the Grignard reagents, which are useful for producing Sn-C bonds. The area remains rich with many applications in industry and continuing activity in the research laboratory.

Tin(II) hydroxide, Sn(OH)2, also known as stannous hydroxide, is an inorganic compound tin(II). The only related material for which definitive information is available is the oxy hydroxide Sn6O4(OH)4, but other related materials are claimed. They are all white solids that are insoluble in water.

Tetrabutyltin (also tetra-n-butyltin and tetra-n-butylstannane) is a stable organotin compound and combustible, colourless liquid at room temperature. It is incompatible with strong oxidizing agents. It has the molecular formula C16H36Sn. Sometimes the abbreviation SnBu4 and TTBT are used.
Tin(II) bromide is a chemical compound of tin and bromine with a chemical formula of SnBr2. Tin is in the +2 oxidation state. The stability of tin compounds in this oxidation state is attributed to the inert pair effect.
Stephen aldehyde synthesis, a named reaction in chemistry, was invented by Henry Stephen (OBE/MBE). This reaction involves the preparation of aldehydes (R-CHO) from nitriles (R-CN) using tin(II) chloride (SnCl2), hydrochloric acid (HCl) and quenching the resulting iminium salt ([R-CH=NH2]+Cl−) with water (H2O). During the synthesis, ammonium chloride is also produced.

Trimethyltin chloride is an organotin compound with the formula (CH3)3SnCl. It is a white solid that is highly toxic and malodorous. It is susceptible to hydrolysis.

Triphenyltin chloride is an organotin compound with formula Sn(C6H5)3Cl. It is a colourless solid that dissolves in organic solvents. It slowly reacts with water. The main use for this compound is as a fungicide and antifoulant.
Tetraethyltin or tetraethyl tin is a chemical compound with the formula C
8H
20Sn and molecular structure (CH3CH2)4Sn, that is, a tin atom attached to four ethyl groups. It is an important example of an organotin compound, often abbreviated as TET.

Tetramethyltin is an organometallic compound with the formula (CH3)4Sn. This liquid, one of the simplest organotin compounds, is useful for transition-metal mediated conversion of acid chlorides to methyl ketones and aryl halides to aryl methyl ketones. It is volatile and toxic, so care should be taken when using it in the laboratory.
Tin(II) sulfide is a chemical compound of tin and sulfur. The chemical formula is SnS. Its natural occurrence concerns herzenbergite (α-SnS), a rare mineral. At elevated temperatures above 905K, SnS undergoes a second order phase transition to β-SnS (space group: cmcm, No. 63). in recent years, it has become evident that a new polymorph of SnS exist based upon the cubic crystal system, π-SnS (space group: P213, No. 198).
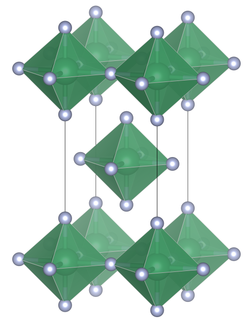
Tin(IV) fluoride is a chemical compound of tin and fluorine with the chemical formula SnF4 and is a white solid with a melting point above 700 °C.
Triphenyltin compounds are organotin compounds with the general formula (C6H5)3SnX. They contain the triphenyltin group, (C6H5)3Sn, or Ph3Sn, which consists of an atom of tin bonded to three phenyl groups. Examples of triphenyltins include:
In chemistry, redistribution usually refers to the exchange of anionic ligands bonded to metal and metalloid centers. The conversion does not involve redox, in contrast to disproportionation reactions. Some useful redistribution reactions are conducted at higher temperatures; upon cooling the mixture, the product mixture is kinetically frozen and the individual products can be separated. In cases where redistribution is rapid at mild temperatures, the reaction is less useful synthetically but still important mechanistically.
Tetrachloride may refer to:









