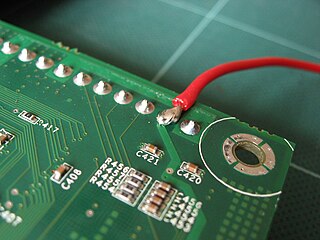
Solder is a fusible metal alloy used to create a permanent bond between metal workpieces. The word solder comes from the Middle English word soudur, via Old French solduree and soulder, from the Latin solidare, meaning "to make solid". In fact, solder must first be melted in order to adhere to and connect the pieces together after cooling, which requires that an alloy suitable for use as solder have a lower melting point than the pieces being joined. The solder should also be resistant to oxidative and corrosive effects that would degrade the joint over time. Solder used in making electrical connections also needs to have favorable electrical characteristics.

Tin is a chemical element with the symbol Sn (from Latin: stannum) and atomic number 50. It is a post-transition metal in group 14 of the periodic table of elements. It is obtained chiefly from the mineral cassiterite, which contains stannic oxide, SnO2. Tin shows a chemical similarity to both of its neighbors in group 14, germanium and lead, and has two main oxidation states, +2 and the slightly more stable +4. Tin is the 49th most abundant element and has, with 10 stable isotopes, the largest number of stable isotopes in the periodic table, thanks to its magic number of protons. It has two main allotropes: at room temperature, the stable allotrope is β-tin, a silvery-white, malleable metal, but at low temperatures it transforms into the less dense grey α-tin, which has the diamond cubic structure. Metallic tin does not easily oxidize in air.

The carbon group is a periodic table group consisting of carbon (C), silicon (Si), germanium (Ge), tin (Sn), lead (Pb), and flerovium (Fl).

Stannite is a mineral, a sulfide of copper, iron, and tin.

Teallite is a sulfide mineral of tin and lead with chemical formula: PbSnS2. It occurs in hydrothermal veins and is sometimes mined as an ore of tin. Teallite forms soft silvery grey mica-like plates and crystallizes in the orthorhombic system. The Mohs hardness is 1.5 to 2 and the specific gravity is 6.4.

Tin(II) chloride, also known as stannous chloride, is a white crystalline solid with the formula SnCl2. It forms a stable dihydrate, but aqueous solutions tend to undergo hydrolysis, particularly if hot. SnCl2 is widely used as a reducing agent (in acid solution), and in electrolytic baths for tin-plating. Tin(II) chloride should not be confused with the other chloride of tin; tin(IV) chloride or stannic chloride (SnCl4).

Tin(II) oxide is a compound with the formula SnO. It is composed of tin and oxygen where tin has the oxidation state of +2. There are two forms, a stable blue-black form and a metastable red form.

Sperrylite is a platinum arsenide mineral with formula PtAs2 and is an opaque metallic tin white mineral which crystallizes in the isometric system with the pyrite group structure. It forms cubic, octahedral or pyritohedral crystals in addition to massive and reniform habits. It has a Mohs hardness of 6 - 7 and a very high specific gravity of 10.6.

Tin(IV) Oxide, also known as stannic oxide, is the inorganic compound with the formula SnO2. The mineral form of SnO2 is called cassiterite, and this is the main ore of tin. With many other names, this oxide of tin is an important material in tin chemistry. It is a colourless, diamagnetic, amphoteric solid.

Argyrodite is an uncommon silver germanium sulfide mineral with formula Ag8GeS6. The color is iron-black with a purplish tinge, and the luster metallic.

Canfieldite is a rare silver tin sulfide mineral with formula: Ag8SnS6. The mineral typically contains variable amounts of germanium substitution in the tin site and tellurium in the sulfur site. There is a complete series between canfieldite and its germanium analogue, argyrodite. It forms black orthorhombic crystals which often appear to be cubic in form due to twinning. The most typical form is as botryoidal rounded grape-like masses. Its Mohs hardness is 2.5 and the specific gravity is 6.28. Canfieldite exhibits conchoidal fracturing and no cleavage.
Tin selenide, also known as stannous selenide, is an inorganic compound with the formula (SnSe), where Tin has a +2 oxidation state. Tin(II) selenide is a narrow band-gap (IV-VI) semiconductor and has received considerable interest for applications including low-cost photovoltaics and memory-switching devices. Tin(II) selenide is a typical layered metal chalcogenide; that is, it includes a Group 16 anion (Se2−) and an electropositive element (Sn2+), and it is arranged in a layered structure.

Tin(II) sulfate (SnSO4) is a chemical compound. It is a white solid that can absorb enough moisture from the air to become fully dissolved, forming an aqueous solution; this property is known as deliquescence. It can be prepared by a displacement reaction between metallic tin and copper(II) sulfate:
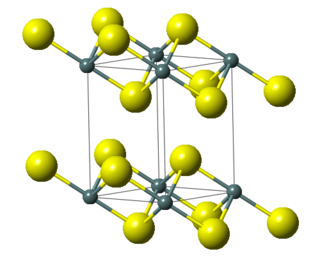
Tin(IV) sulfide is a compound with the formula SnS
2. The compound crystallizes in the cadmium iodide motif, with the Sn(IV) situated in "octahedral holes' defined by six sulfide centers. It occurs naturally as the rare mineral berndtite. It is useful as semiconductor material with band gap 2.2 eV.
Lead(IV) sulfide is a chemical compound with the formula PbS2. This material is generated by the reaction of the more common lead(II) sulfide, PbS, with sulfur at >600 °C and at high pressures. PbS2, like the related tin(IV) sulfide SnS2, crystallises in the cadmium iodide motif, which indicates that Pb should be assigned the formal oxidation state of 4+.
Tin(II) sulfide is a chemical compound of tin and sulfur. The chemical formula is SnS. Its natural occurrence concerns herzenbergite (α-SnS), a rare mineral. At elevated temperatures above 905K, SnS undergoes a second order phase transition to β-SnS (space group: cmcm, No. 63). in recent years, it has become evident that a new polymorph of SnS exist based upon the cubic crystal system, π-SnS (space group: P213, No. 198).
Stannoidite is a sulfide mineral composed of five chemical elements: copper, iron, zinc, tin and sulfur. Its name originates from Latin stannum (tin) and Greek eides. The mineral is found in hydrothermal Cu-Sn deposits.

Kesterite is a sulfide mineral with a formula Cu2(Zn,Fe)SnS4. In its lattice structure, zinc and iron atoms share the same lattice sites. Kesterite is the Zn-rich variety whereas the Zn-poor form is called ferrokesterite or stannite. Owing to their similarity, kesterite is sometimes called isostannite. The synthetic form of kesterite is abbreviated as CZTS (from copper zinc tin sulfide). The name kesterite is sometimes extended to include this synthetic material and also CZTSe, which contains selenium instead of sulfur.














