This page is based on this
Wikipedia article Text is available under the
CC BY-SA 4.0 license; additional terms may apply.
Images, videos and audio are available under their respective licenses.

Tryptophan synthase or tryptophan synthetase is an enzyme that catalyzes the final two steps in the biosynthesis of tryptophan. It is commonly found in Eubacteria, Archaebacteria, Protista, Fungi, and Plantae. However, it is absent from Animalia. It is typically found as an α2β2 tetramer. The α subunits catalyze the reversible formation of indole and glyceraldehyde-3-phosphate (G3P) from indole-3-glycerol phosphate (IGP). The β subunits catalyze the irreversible condensation of indole and serine to form tryptophan in a pyridoxal phosphate (PLP) dependent reaction. Each α active site is connected to a β active site by a 25 angstrom long hydrophobic channel contained within the enzyme. This facilitates the diffusion of indole formed at α active sites directly to β active sites in a process known as substrate channeling. The active sites of tryptophan synthase are allosterically coupled.

L-Kynurenine is a metabolite of the amino acid L-tryptophan used in the production of niacin.
Catechol dioxygenases are metalloprotein enzymes that carry out the oxidative cleavage of catechols. This class of enzymes incorporate dioxygen into the substrate (biochemistry). Catechol dioxygenases belong to the class of oxidoreductases and have several different substrate specificities, including catechol 1,2-dioxygenase, catechol 2,3-dioxygenase, and protocatechuate 3,4-dioxygenase. The active site of catechol dioxygenases most frequently contains iron, but manganese-containing forms are also known.

Catechol 1,2- dioxygenase is an enzyme that catalyzes the oxidative ring cleavage of catechol to form cis,cis-muconic acid:
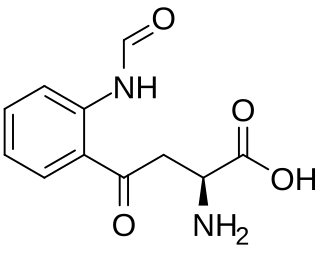
N′-Formylkynurenine is an intermediate in the catabolism of tryptophan. It is a formylated derivative of kynurenine. The formation of N′-formylkynurenine is catalyzed by heme dioxygenases.
In enzymology, a tryptophan alpha,beta-oxidase (EC 1.3.3.10) is an enzyme that catalyzes the chemical reaction
In enzymology, a 2,3-dihydroxyindole 2,3-dioxygenase (EC 1.13.11.23) is an enzyme that catalyzes the chemical reaction

In enzymology, a 3-hydroxyanthranilate 3,4-dioxygenase (EC 1.13.11.6) is an enzyme that catalyzes the chemical reaction
In enzymology, a 7,8-dihydroxykynurenate 8,8a-dioxygenase (EC 1.13.11.10) is an enzyme that catalyzes the chemical reaction
In enzymology, an indole 2,3-dioxygenase (EC 1.13.11.17) is an enzyme that catalyzes the chemical reaction
In enzymology, a peptide-tryptophan 2,3-dioxygenase (EC 1.13.11.26) is an enzyme that catalyzes the chemical reaction
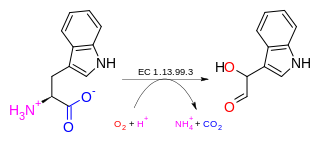
In enzymology, a tryptophan 2'-dioxygenase is an enzyme that catalyzes the chemical reaction

Dioxygenases are oxidoreductase enzymes. Aerobic life, from simple single-celled bacteria species to complex eukaryotic organisms, has evolved to depend on the oxidizing power of dioxygen in various metabolic pathways. From energetic adenosine triphosphate (ATP) generation to xenobiotic degradation, the use of dioxygen as a biological oxidant is widespread and varied in the exact mechanism of its use. Enzymes employ many different schemes to use dioxygen, and this largely depends on the substrate and reaction at hand.

The kynurenine pathway is a metabolic pathway leading to the production of nicotinamide adenine dinucleotide (NAD+) from the degradation of the essential amino acid tryptophan. Disruption in the pathway is associated with certain genetic disorders.
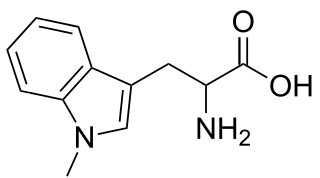
1-Methyltryptophan is a chemical compound that is an inhibitor of the tryptophan catabolic enzyme indoleamine 2,3-dioxygenase. It is a chiral compound that can exist as both D- and L-isomers (enantiomers).

Indoleamine 2,3-dioxygenase 2 (IDO2) is a protein that in humans is encoded by the IDO2 gene.










