
In chemistry, a salt is an ionic compound that can be formed by the neutralization reaction of an acid and a base. Salts are composed of related numbers of cations and anions so that the product is electrically neutral. These component ions can be inorganic, such as chloride (Cl−), or organic, such as acetate ; and can be monatomic, such as fluoride (F−), or polyatomic, such as sulfate.

An acetate is a salt formed by the combination of acetic acid with an alkaline, earthy, metallic or nonmetallic and other base. "Acetate" also describes the conjugate base or ion typically found in aqueous solution and written with the chemical formula C
2H
3O−
2. The neutral molecules formed by the combination of the acetate ion and a positive ion are also commonly called "acetates". The simplest of these is hydrogen acetate with corresponding salts, esters, and the polyatomic anion CH
3CO−
2, or CH
3COO−
.
Classical qualitative inorganic analysis is a method of analytical chemistry which seeks to find the elemental composition of inorganic compounds. It is mainly focused on detecting ions in an aqueous solution, therefore materials in other forms may need to be brought to this state before using standard methods. The solution is then treated with various reagents to test for reactions characteristic of certain ions, which may cause color change, precipitation and other visible changes.
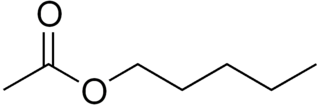
Amyl acetate (pentyl acetate) is an organic compound and an ester with the chemical formula CH3COO[CH2]4CH3 and the molecular weight 130.19 g/mol. It has a scent similar to pears and apples. The compound is the condensation product of acetic acid and 1-pentanol. However, esters formed from other pentanol isomers (amyl alcohols), or mixtures of pentanols, are often referred to as amyl acetate.

Zinc acetate is a salt with the formula Zn(CH3CO2)2, which commonly occurs as the dihydrate Zn(CH3CO2)2·2H2O. Both the hydrate and the anhydrous forms are colorless solids that are commonly used in chemical synthesis and as dietary supplements. Zinc acetates are prepared by the action of acetic acid on zinc carbonate or zinc metal. When used as a food additive, it has the E number E650.

Calcium acetate is a chemical compound which is a calcium salt of acetic acid. It has the formula Ca(C2H3O2)2. Its standard name is calcium acetate, while calcium ethanoate is the systematic name. An older name is acetate of lime. The anhydrous form is very hygroscopic; therefore the monohydrate (Ca(CH3COO)2•H2O) is the common form.

Lead(II) carbonate is the chemical compound PbCO3. It is prepared industrially from lead(II) acetate and carbon dioxide.

Organolead compounds are chemical compounds containing a chemical bond between carbon and lead. Organolead chemistry is the corresponding science. The first organolead compound was hexaethyldilead (Pb2(C2H5)6), first synthesized in 1858. Sharing the same group with carbon, lead is tetravalent.

Cobalt(II) acetate is the cobalt salt of acetic acid. It is commonly found as the tetrahydrate Co(CH3CO2)2·4 H2O, abbreviated Co(OAc)2·4 H2O. It is used as a catalyst.

Acetic acid, systematically named ethanoic acid, is a colourless liquid organic compound with the chemical formula CH3COOH (also written as CH3CO2H or C2H4O2). When undiluted, it is sometimes called glacial acetic acid. Vinegar is no less than 4% acetic acid by volume, making acetic acid the main component of vinegar apart from water. Acetic acid has a distinctive sour taste and pungent smell. In addition to household vinegar, it is mainly produced as a precursor to polyvinyl acetate and cellulose acetate. It is classified as a weak acid since it only partially dissociates in solution, but concentrated acetic acid is corrosive and can attack the skin.

Lead(II) thiocyanate is a compound, more precisely a salt, with the formula Pb(SCN)2.It is a white crystalline solid, but will turn yellow upon exposure to light. It is soluble in water and can be converted to a basic salt (Pb(CNS)2*Pb(OH)2 when boiled. Salt crystals may form upon cooling. Lead thiocyanate can cause lead poisoning if ingested and can adversely react with many substances. It has use in small explosives, matches, and dyeing.
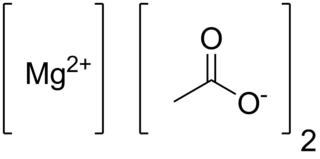
Anhydrous magnesium acetate has the chemical formula Mg(C2H3O2)2 and in its hydrated form, magnesium acetate tetrahydrate, it has the chemical formula Mg(CH3COO)2 • 4H2O. In this compound magnesium has an oxidation state of 2+. Magnesium acetate is the magnesium salt of acetic acid. It is deliquescent and upon heating, it decomposes to form magnesium oxide. Magnesium acetate is commonly used as a source of magnesium in biological reactions.

Barium acetate (Ba(C2H3O2)2) is the salt of barium(II) and acetic acid.
Lead carbide is a hypothetical chemical compound of carbon and lead. Lead and elemental carbon do not normally combine, even at very high temperatures. Modern literature on lead carbide is almost non-existent.
Lead(II) phosphate is an ionic compound with chemical formula Pb3(PO4)2. Lead(II) phosphate is a long-lived electronically neutral reagent chemical. Despite limited tests on humans, it has been identified as a carcinogen based on tests on animals conducted by the EPA. Lead(II) phosphate appears as hexagonal, colorless crystals or as a white powder. Lead(II) phosphate is insoluble in water and alcohol but soluble in HNO3 and has fixed alkali hydroxides. When lead(II) phosphate is heated for decomposition it emits very toxic fumes containing Pb and POx.

Nickel(II) acetate is the name for the coordination compounds with the formula Ni(CH3CO2)2·x H2O where x can be 0, 2, and 4. The green tetrahydrate Ni(CH3CO2)2·4 H2O is most common. It is used for electroplating.
Aluminium triacetate, formally named aluminium acetate, is a chemical compound with composition Al(CH
3CO
2)
3. Under standard conditions it appears as a white, water-soluble solid that decomposes on heating at around 200 °C. The triacetate hydrolyses to a mixture of basic hydroxide / acetate salts, and multiple species co-exist in chemical equilibrium, particularly in aqueous solutions of the acetate ion; the name aluminium acetate is commonly used for this mixed system.
Aluminium sulfacetate is a chemical compound of aluminium with formula Al
2SO
4(CH
3CO
2)
4.














