
In physics and thermodynamics, an equation of state is a thermodynamic equation relating state variables which describe the state of matter under a given set of physical conditions, such as pressure, volume, temperature (PVT), or internal energy. Equations of state are useful in describing the properties of fluids, mixtures of fluids, solids, and the interior of stars.
Relative density, or specific gravity, is the ratio of the density of a substance to the density of a given reference material. Specific gravity usually means relative density with respect to water. The term "relative density" is often preferred in scientific usage. It is defined as a ratio of density of particular substance with that of water.

In celestial mechanics, the Roche limit, also called Roche radius, is the distance within which a celestial body, held together only by its own gravity, will disintegrate due to a second celestial body's tidal forces exceeding the first body's gravitational self-attraction. Inside the Roche limit, orbiting material disperses and forms rings whereas outside the limit material tends to coalesce. The term is named after Édouard Roche, who was the French astronomer who first calculated this theoretical limit in 1848.
In viscous fluid dynamics, the Archimedes number (Ar), named after the ancient Greek scientist Archimedes is used to determine the motion of fluids due to density differences. It is a dimensionless number, the ratio of gravitational forces to viscous forces and has the form:

Specific gravity is the ratio of the density of a substance to the density of a reference substance; equivalently, it is the ratio of the mass of a substance to the mass of a reference substance for the same given volume. Apparent specific gravity is the ratio of the weight of a volume of the substance to the weight of an equal volume of the reference substance. The reference substance for liquids is nearly always water at its densest ; for gases it is air at room temperature. Nonetheless, the temperature and pressure must be specified for both the sample and the reference. Pressure is nearly always 1 atm (101.325 kPa).
The density of air or atmospheric density, denoted ρ, is the mass per unit volume of Earth's atmosphere. Air density, like air pressure, decreases with increasing altitude. It also changes with variation in atmospheric pressure, temperature and humidity. At 1013,25 hPa (abs) and 15°C, air has a density of approximately 1.225 kg/m³ according to ISA.

In classical electromagnetism, polarization density is the vector field that expresses the density of permanent or induced electric dipole moments in a dielectric material. When a dielectric is placed in an external electric field, its molecules gain electric dipole moment and the dielectric is said to be polarized. The electric dipole moment induced per unit volume of the dielectric material is called the electric polarization of the dielectric.

A slurry is a mixture of solids with specific gravity greater than 1 suspended in liquid, usually water. Most common use of slurry is as a mean of transportation of solids being the liquid a carrier that is pumped on a device like a centrifugal pump. The size of solid particles may vary from 1 micron up to hundred of milliliters. The solids may settle at certain transport velocity and the mixture can behave as Newtonian or non-Newtonian fluid. Depending on the mixture the slurry may be abrasive and corrosive.
The ideal gas law can be stated in terms of the compressibility factor Z:
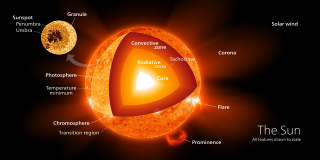
A radiation zone, or radiative region is a layer of a star's interior where energy is primarily transported toward the exterior by means of radiative diffusion and thermal conduction, rather than by convection. Energy travels through the radiation zone in the form of electromagnetic radiation as photons.
A wet gas is any gas with a small amount of liquid present. The term "wet gas" has been used to describe a range of conditions varying from a humid gas which is gas saturated with liquid vapour to a multiphase flow with a 90% volume of gas. There has been some debate as to its actual definition but there is currently no fully defined quantitative definition of a wet gas flow that is universally accepted.
The Ostwald–Freundlich equation governs boundaries between two phases; specifically, it relates the surface tension of the boundary to its curvature, the ambient temperature, and the vapor pressure or chemical potential in the two phases.
Accidental release source terms are the mathematical equations that quantify the flow rate at which accidental releases of liquid or gaseous pollutants into the ambient environment can occur at industrial facilities such as petroleum refineries, petrochemical plants, natural gas processing plants, oil and gas transportation pipelines, chemical plants, and many other industrial activities. Governmental regulations in many countries require that the probability of such accidental releases be analyzed and their quantitative impact upon the environment and human health be determined so that mitigating steps can be planned and implemented.

In thermodynamics, vapour quality is the mass fraction in a saturated mixture that is vapour; in other words, saturated vapour has a "quality" of 100%, and saturated liquid has a "quality" of 0%. Vapour quality is an intensive property which can be used in conjunction with other independent intensive properties to specify the thermodynamic state of the working fluid of a thermodynamic system. It has no meaning for substances which are not saturated mixtures . Vapor quality is an important quantity during the adiabatic expansion step in various thermodynamic cycles. Working fluids can be classified by using the appearance of droplets in the vapour during the expansion step.
The Mie–Grüneisen equation of state is a relation between the pressure and the volume of a solid at a given temperature. It is used to determine the pressure in a shock-compressed solid. The Mie–Grüneisen relation is a special form of the Grüneisen model which describes the effect that changing the volume of a crystal lattice has on its vibrational properties. Several variations of the Mie–Grüneisen equation of state are in use.
The characterization gas collecting tube describes an oblong gas-tight container with one valve at either end. Usually such a container has a gauged volume, has a cylindrical shape and is made of glass. Gas collecting tubes are used for science-related purposes; for taking samples of gases.
The vaporizing droplet problem is a challenging issue in fluid dynamics. It is part of many engineering situations involving the transport and computation of sprays: fuel injection, spray painting, aerosol spray, flashing releases… In most of these engineering situations there is a relative motion between the droplet and the surrounding gas. The gas flow over the droplet has many features of the gas flow over a rigid sphere: pressure gradient, viscous boundary layer, wake. In addition to these common flow features one can also mention the internal liquid circulation phenomenon driven by surface-shear forces and the boundary layer blowing effect.
Slip ratio in gas–liquid (two-phase) flow, is defined as the ratio of the velocity of the gas phase to the velocity of the liquid phase.
The flow in many fluids varies with density and depends upon the gravity. Due to which the fluid with lower density is always above the fluid with higher density. Stratified flows are very common such as the Earth's ocean and its atmosphere.












