This page is based on this
Wikipedia article Text is available under the
CC BY-SA 4.0 license; additional terms may apply.
Images, videos and audio are available under their respective licenses.
Free-radical addition is an addition reaction in organic chemistry involving free radicals. The addition may occur between a radical and a non-radical, or between two radicals.
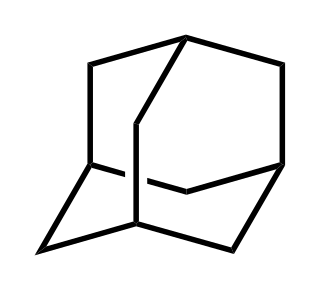
Adamantane is a colorless, crystalline chemical compound with a camphor-like odor. With a formula C10H16, it is a cycloalkane and also the simplest diamondoid. Adamantane molecules consists of three connected cyclohexane rings arranged in the "armchair" configuration. It is unique in that it is both rigid and virtually stress-free. Adamantane is the most stable among all the isomers with formula C10H16, which include the somewhat similar twistane. The spatial arrangement of carbon atoms in the adamantane molecule is the same as in the diamond crystal. This motivates the name adamantane, which is derived from the Greek adamantinos (relating to steel or diamond).
A rearrangement reaction is a broad class of organic reactions where the carbon skeleton of a molecule is rearranged to give a structural isomer of the original molecule. Often a substituent moves from one atom to another atom in the same molecule. In the example below the substituent R moves from carbon atom 1 to carbon atom 2:
A 1,2-rearrangement or 1,2-migration or 1,2-shift or Whitmore 1,2-shift is an organic reaction where a substituent moves from one atom to another atom in a chemical compound. In a 1,2 shift the movement involves two adjacent atoms but moves over larger distances are possible. In the example below the substituent R moves from carbon atom C2 to C3.

Organic reductions or organic oxidations or organic redox reactions are redox reactions that take place with organic compounds. In organic chemistry oxidations and reductions are different from ordinary redox reactions because many reactions carry the name but do not actually involve electron transfer in the electrochemical sense of the word. Instead the relevant criterion for organic oxidation is gain of oxygen and/or loss of hydrogen
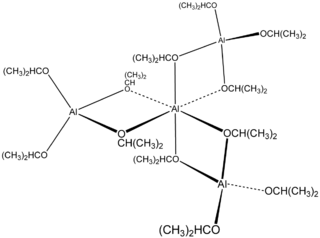
Aluminium isopropoxide is the chemical compound usually described with the formula Al(O-i-Pr)3, where i-Pr is the isopropyl group (–CH(CH3)2). This colourless solid is a useful reagent in organic synthesis. The structure of this compound is complex, possibly time-dependent, and may depend on solvent.
Neighbouring group participation (NGP) in organic chemistry has been defined by IUPAC as the interaction of a reaction centre with a lone pair of electrons in an atom or the electrons present in a sigma bond or pi bond contained within the parent molecule but not conjugated with the reaction centre. When NGP is in operation it is normal for the reaction rate to be increased. It is also possible for the stereochemistry of the reaction to be abnormal when compared with a normal reaction. While it is possible for neighbouring groups to influence many reactions in organic chemistry this page is limited to neighbouring group effects seen with carbocations and SN2 reactions.

Triethyloxonium tetrafluoroborate is the organic oxonium compound with the formula [(CH3CH2)3O]BF4. It is often called Meerwein's Reagent or Meerwein's Salt after its discoverer Hans Meerwein. Also well known and commercially available is the related trimethyloxonium tetrafluoroborate. The compounds are white solids that dissolve in polar organic solvents. They are strong alkylating agents. Aside from the BF−
4 salt, many related derivatives are available.
Oppenauer oxidation, named after Rupert Viktor Oppenauer, is a gentle method for selectively oxidizing secondary alcohols to ketones.

Hans Meerwein was a German chemist.
Several reactions and reagents bear his name, most notably the Meerwein–Ponndorf–Verley reduction, the Wagner–Meerwein rearrangement, the Meerwein arylation reaction, and Meerwein's salt.
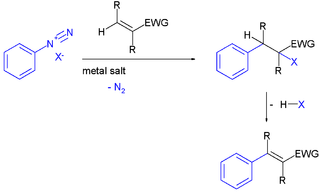
An aryl radical in organic chemistry is a reactive intermediate and an arene compound incorporating one free radical carbon atom as part of the ring structure. As such it is the radical counterpart of the Arenium ion. The parent compound is the phenyl radical C6H5.. Aryl radicals are intermediates in certain organic reactions.

Trimethyloxonium tetrafluoroborate is the organic compound with the formula Me3OBF4. It is sometimes called the "Meerwein salt" after Hans Meerwein.) This salt is a strong methylating agent, being a synthetic equivalent of CH3(+). It is a white solid that rapidly degrades upon exposure to atmospheric moisture, although it is robust enough to be weighed and dispensed quickly without the benefit of inert atmosphere protection. Triethyloxonium tetrafluoroborate is a closely related reagent.

Cericlamine (INN) is a potent and moderately selective serotonin reuptake inhibitor (SSRI) of the amphetamine family that was investigated as an antidepressant for the treatment of depression, anxiety disorders, and anorexia nervosa by Jouveinal but did not complete development and was never marketed. It reached phase III clinical trials in 1996 before development was discontinued in 1999.

2,2-Diethoxytetrahydrofuran is a cyclic orthoester which can be reacted with diols to biodegradable polyorthoesters.

Taraxasterol (anthesterin) is a triterpene derived from the mevalonate pathway and is found in dandelions.

Igor Igorevich Wagner was a Russian chemist.