Organolithium reagents are organometallic compounds that contain carbon – lithium bonds. They are important reagents in organic synthesis, and are frequently used to transfer the organic group or the lithium atom to the substrates in synthetic steps, through nucleophilic addition or simple deprotonation. Organolithium reagents are used in industry as an initiator for anionic polymerization, which leads to the production of various elastomers. They have also been applied in asymmetric synthesis in the pharmaceutical industry. Due to the large difference in electronegativity between the carbon atom and the lithium atom, the C-Li bond is highly ionic. Owing to the polar nature of the C-Li bond, organolithium reagents are good nucleophiles and strong bases. For laboratory organic synthesis, many organolithium reagents are commercially available in solution form. These reagents are highly reactive, and are sometimes pyrophoric.

Pentalene is a polycyclic hydrocarbon composed of two fused cyclopentadiene rings. It has chemical formula C8H6. It is antiaromatic, because it has 4n π electrons where n is any integer. For this reason it dimerizes even at temperatures as low as −100 °C. The derivative 1,3,5-tri-tert-butylpentalene was synthesized in 1973. Because of the tert-butyl substituents this compound is thermally stable. Pentalenes can also be stabilized by benzannulation for example in the compounds benzopentalene and dibenzopentalene.
Acetylide refers to chemical compounds with the chemical formulas MC≡CH and MC≡CM, where M is a metal. The term is used loosely and can refer to substituted acetylides having the general structure RC≡CM. Acetylides are reagents in organic synthesis. The calcium acetylide commonly called calcium carbide is a major compound of commerce.
The Wittig reaction or Wittig olefination is a chemical reaction of an aldehyde or ketone with a triphenyl phosphonium ylide to give an alkene and triphenylphosphine oxide.
As the name suggests, a non-nucleophilic base is a sterically hindered organic base that is a poor nucleophile. Normal bases are also nucleophiles, but often chemists seek the proton-removing ability of a base without any other functions. Typical non-nucleophilic bases are bulky, such that protons can attach to the basic center but alkylation and complexation is inhibited.

tert-Butyl alcohol (TBA), also called tert-butanol or t-butanol, is the simplest tertiary alcohol, with a formula of (CH3)3COH (sometimes represented as t-BuOH). It is one of the four isomers of butanol. tert-Butyl alcohol is a colorless solid, which melts near room temperature and has a camphor-like odor. It is miscible with water, ethanol and diethyl ether.
The Corey–House synthesis (also called the Corey–Posner–Whitesides–House reaction and other permutations) is an organic reaction that involves the reaction of a lithium diorganylcuprate (R2CuLi) with an organyl (pseudo)halide (R'X) to form a new alkane, as well as an ill-defined organocopper species and lithium halide as byproducts.

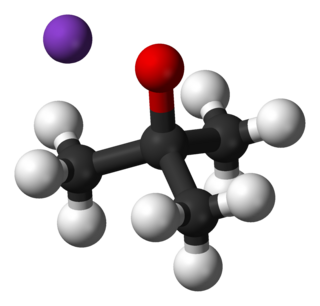
tert-Butyllithium is a chemical compound with the formula (CH3)3CLi. As an organolithium compound, it has applications in organic synthesis since it is a strong base, capable of deprotonating many carbon acids, including benzene. tert-Butyllithium is available commercially as hydrocarbon solutions; it is not usually prepared in the laboratory. Its synthesis was first reported by R. B. Woodward in 1941.

Directed ortho metalation (DoM) is an adaptation of electrophilic aromatic substitution in which electrophiles attach themselves exclusively to the ortho- position of a direct metalation group or DMG through the intermediary of an aryllithium compound. The DMG interacts with lithium through a hetero atom. Examples of DMG's are the methoxy group, a tertiary amine group and an amide group.

Organozinc compounds in organic chemistry contain carbon to zinc chemical bonds. Organozinc chemistry is the science of organozinc compounds describing their physical properties, synthesis and reactions.
Oppenauer oxidation, named after Rupert Viktor Oppenauer, is a gentle method for selectively oxidizing secondary alcohols to ketones.
Phosphazenes are a class of chemical compounds in which a phosphorus atom is covalently linked to a nitrogen atom by a double bond and to three other atoms or radicals by single bonds. While other substitutions produce relatively persistent compounds, in organic synthesis the term largely refers to species with three amino substituents bound to phosphorus. The compounds are unusually stable examples of the phosphorane class of molecules and have a remarkable proton affinity. As such, they are one of the eminent examples of neutral, organic superbases. Two examples are hexachlorocyclotriphosphazene and bis(triphenylphosphine)iminium chloride. Phosphazenes are also known as iminophosphoranes and phosphine imides.

The group 2 elements are known to form organometallic compounds. Of these, organomagnesium compounds, usually in the form of Grignard reagents are widely used in organic chemistry, while the other organometallic compounds of this group are largely academic.
Organosodium chemistry is the chemistry of organometallic compounds containing a carbon to sodium chemical bond. The application of organosodium compounds in chemistry is limited in part due to competition from organolithium compounds, which are commercially available and exhibit more convenient reactivity.
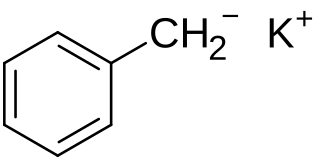
Benzyl potassium is an organopotassium compound with the formula C6H5CH2K that takes the form of an air sensitive orange powder. Like organo-alkali metal reagents in general, benzyl potassium is highly reactive, so much so that its use in coordinating solvents such as ethers and amines is less common than in hydrocarbons, as gradual decomposition occurs.

P4-t-Bu is a readily accessible chemical from the group of neutral, peralkylated sterically hindered polyaminophosphazenes, which are extremely strong bases but very weak nucleophiles. P4-t-Bu can also be regarded as tetrameric triaminoiminophosphorane of the basic structure (H2N)3P=N-H. The homologous series of P1 to P7 polyaminophosphazenes of the general formula with preferably methyl groups as R1, a methyl group or tert.-butyl group as and even-numbered x between 0 and 6 (P4-t-Bu: R1 = Me, R2 = t-Bu und x = 3) has been developed by Reinhard Schwesinger; the resulting phosphazene bases are therefore also referred to as Schwesinger superbases.
n-Butylsodium CH3CH2CH2CH2Na is an organometallic compound containing sodium ions and butyl anions, which can be used to add sodium to extremely weak organic acids.