Ilmenite, also known as manaccanite, is a titanium-iron oxide mineral with the idealized formula FeTiO
3. It is a weakly magnetic black or steel-gray solid. From a commercial perspective, ilmenite is the most important ore of titanium. Ilmenite is the main source of titanium dioxide, which is used in paints, printing inks, fabrics, plastics, paper, sunscreen, food and cosmetics.
Titanic acid is a general name for a family of chemical compounds of the elements titanium, hydrogen, and oxygen, with the general formula [TiOx(OH)4−2x]n. Various simple titanic acids have been claimed, mainly in the older literature. No crystallographic and little spectroscopic support exists for these materials. Some older literature including Brauer's Handbook refers to TiO2 as titanic acid.

Titanium dioxide, also known as titanium(IV) oxide or titania, is the naturally occurring oxide of titanium, chemical formula TiO
2. When used as a pigment, it is called titanium white, Pigment White 6 (PW6), or CI 77891. Generally, it is sourced from ilmenite, rutile and anatase. It has a wide range of applications, including paint, sunscreen and food coloring. When used as a food coloring, it has E number E171. World production in 2014 exceeded 9 million metric tons. It has been estimated that titanium dioxide is used in two-thirds of all pigments, and the oxide has been valued at $13.2 billion.

Titanium tetrachloride is the inorganic compound with the formula TiCl4. It is an important intermediate in the production of titanium metal and the pigment titanium dioxide. TiCl4 is a volatile liquid. Upon contact with humid air, it forms spectacular opaque clouds of titanium dioxide (TiO2) and hydrated hydrogen chloride. It is sometimes referred to as "tickle" or "tickle 4" due to the phonetic resemblance of its molecular formula (TiCl4) to the word.
The Kroll process is a pyrometallurgical industrial process used to produce metallic titanium. It was invented in 1940 by William J. Kroll in Luxembourg. After moving to the United States, Kroll further developed the method for the production of zirconium. The Kroll process replaced the Hunter process for almost all commercial production.
Titanium alloys are metals that contain a mixture of titanium and other chemical elements. Such alloys have very high tensile strength and toughness. They are light in weight, have extraordinary corrosion resistance and the ability to withstand extreme temperatures. However, the high cost of both raw materials and processing limit their use to military applications, aircraft, spacecraft, bicycles, medical devices, jewelry, highly stressed components such as connecting rods on expensive sports cars and some premium sports equipment and consumer electronics.

The Petasis reagent is an organotitanium compound with the formula Cp2Ti(CH3)2. It is an orange-colored solid.

Titanium tetraiodide is an inorganic compound with the formula TiI4. It is a black volatile solid, first reported by Rudolph Weber in 1863. It is an intermediate in the Van Arkel process for the purification of titanium.

Titanium hydride normally refers to the inorganic compound TiH2 and related nonstoichiometric materials. It is commercially available as a stable grey/black powder, which is used as an additive in the production of Alnico sintered magnets, in the sintering of powdered metals, the production of metal foam, the production of powdered titanium metal and in pyrotechnics.

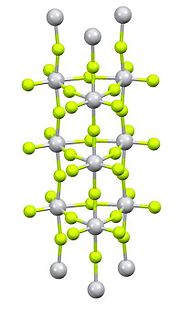
Titanium disulfide is an inorganic compound with the formula TiS2. A golden yellow solid with high electrical conductivity, it belongs to a group of compounds called transition metal dichalcogenides, which consist of the stoichiometry ME2. TiS2 has been employed as a cathode material in rechargeable batteries.
Titanium(IV) hydride is an inorganic compound with the empirical chemical formula TiH
4. It has not yet been obtained in bulk, hence its bulk properties remain unknown. However, molecular titanium(IV) hydride has been isolated in solid gas matrices. The molecular form is a colourless gas, and very unstable toward thermal decomposition. As such the compound is not well characterised, although many of its properties have been calculated via computational chemistry.

Titanium was first introduced into surgeries in the 1950s after having been used in dentistry for a decade prior. It is now the metal of choice for prosthetics, internal fixation, inner body devices, and instrumentation. Titanium is used from head to toe in biomedical implants. One can find titanium in neurosurgery, bone conduction hearing aids, false eye implants, spinal fusion cages, pacemakers, toe implants, and shoulder/elbow/hip/knee replacements along with many more. The main reason why titanium is often used in the body is due to titanium's biocompatibility and, with surface modifications, bioactive surface. The surface characteristics that affect biocompatibility are surface texture, steric hindrance, binding sites, and hydrophobicity (wetting). These characteristics are optimized to create an ideal cellular response. Some medical implants, as well as parts of surgical instruments are coated with titanium nitride (TiN).
Hexafluorotitanic acid (systematically named oxonium hexafluoridotitanate(2-)) is an inorganic compound with the chemical formula (H3O)2[TiF6].
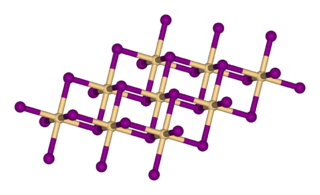
Titanium diselenide (TiSe2) also known as titanium(IV) selenide, is an inorganic compound of titanium and selenium. In this material selenium is viewed as selenide (Se2−) which requires that titanium exists as Ti4+. Titanium diselenide is a member of metal dichalcogenides, compounds that consist of a metal and an element of the chalcogen column within the periodic table. Many exhibit properties of potential value in battery technology, such as intercalation and electrical conductivity, although most applications focus on the less toxic and lighter disulfides, e.g. TiS2.
Titanium(III) bromide is the inorganic compound with the formula TiBr3. It is a blue black paramagnetic solid with a reddish reflection. It has few applications, although it is a catalyst for the polymerization of alkenes.
Titanium(III) iodide is an inorganic compound with the formula TiI3. It is a dark violet solid that is insoluble in solvents, except upon decomposition.