Related Research Articles
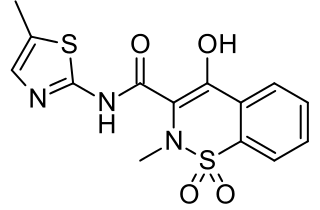
Meloxicam, sold under the brand name Mobic among others, is a nonsteroidal anti-inflammatory drug (NSAID) used to treat pain and inflammation in rheumatic diseases and osteoarthritis. It is used by mouth or by injection into a vein. It is recommended that it be used for as short a period as possible and at a low dose.

Pentosan polysulfate, sold under the brand name Elmiron among others, is a medication used for the treatment of interstitial cystitis. It was approved for medical use in the United States in 1996.
Adalimumab, sold under the brand name Humira and others, is a disease-modifying antirheumatic drug and monoclonal antibody used to treat rheumatoid arthritis, juvenile idiopathic arthritis, psoriatic arthritis, ankylosing spondylitis, Crohn's disease, ulcerative colitis, plaque psoriasis, hidradenitis suppurativa, and uveitis. It is administered by subcutaneous injection. It works by inactivating tumor necrosis factor-alpha (TNFα).

Carprofen is a nonsteroidal anti-inflammatory drug (NSAID) of the carbazole and propionic acid class that was previously for use in humans and animals but is now only available to veterinarians for prescribing as a supportive treatment for various conditions in animals. Carprofen reduces inflammation by inhibition of COX-1 and COX-2; its specificity for COX-2 varies from species to species. Marketed under many brand names worldwide, carprofen is used as a treatment for inflammation and pain, including joint pain and postoperative pain.
Ofatumumab is a fully human monoclonal antibody to CD20, which appears to provide rapid B-cell depletion. Under the brand name Kesimpta, it is approved for the treatment of multiple sclerosis in the United States as well as in the European Union and other regions. Under the brand name Arzerra, it is approved for the treatment of certain types of chronic lymphocytic leukemia (CLL) in the United States. It is sold by Novartis under license from Genmab.
Raxibacumab is a human monoclonal antibody intended for the prophylaxis and treatment of inhaled anthrax. Its efficacy has been proven in rabbits and monkeys. In December 2012 raxibacumab was approved in the United States for the treatment of inhalational anthrax due to Bacillus anthracis in combination with appropriate antibacterial drugs, and for prophylaxis of inhalational anthrax when alternative therapies are not available or are not appropriate.

Maropitant (INN; brand name: Cerenia, used as maropitant citrate (USAN), is a neurokinin-1 (NK1) receptor antagonist developed by Zoetis specifically for the treatment of motion sickness and vomiting in dogs. It was approved by the FDA in 2007, for use in dogs and in 2012, for cats.
Tanezumab is a monoclonal antibody against nerve growth factor as a treatment for pain via a novel mechanisms different from conventional pain-killer drugs. Tanezumab was discovered and developed by Rinat Neuroscience and was acquired by Pfizer in 2006.
Ixekizumab, sold under the brand name Taltz, is an injectable medication for the treatment of autoimmune diseases. Chemically, it is a form of a humanized monoclonal antibody. The substance acts by binding interleukin 17A and neutralizing it, reducing inflammation.
Mogamulizumab, sold under the brand name Poteligeo, is a humanized, afucosylated monoclonal antibody targeting CC chemokine receptor type 4 (CCR4). It is given by injection into a vein.
Calcitonin gene-related peptide (CGRP) receptor antagonists are a class of drugs that act as antagonists of the calcitonin gene-related peptide receptor (CGRPR).

Dupilumab, sold under the brand name Dupixent, is a monoclonal antibody blocking interleukin 4 and interleukin 13, used for allergic diseases such as atopic dermatitis (eczema), asthma and nasal polyps which result in chronic sinusitis. It is also used for the treatment of eosinophilic esophagitis and prurigo nodularis.
Lokivetmab, trade name Cytopoint, is a monoclonal antibody used to treat atopic dermatitis in dogs. It acts against interleukin 31 (IL-31), which is a cytokine involved in causing itchiness (pruritus). Lokivetmab is administered by subcutaneous injection; each dose is effective for four to eight weeks.
Satralizumab, sold under the brand name Enspryng, is a humanized monoclonal antibody medication that is used for the treatment of neuromyelitis optica spectrum disorder (NMOSD), a rare autoimmune disease. The drug is being developed by Chugai Pharmaceutical, a subsidiary of Roche.

Grapiprant, sold under the brand name Galliprant, is a small molecule drug that belongs in the piprant class. This analgesic and anti-inflammatory drug is primarily used as a pain relief for mild to moderate inflammation related to osteoarthritis in dogs. Grapiprant has been approved by the FDA's Center for Veterinary Medicine and was categorized as a non-cyclooxygenase inhibiting non-steroidal anti-inflammatory drug (NSAID) in March 2016.
Bamlanivimab is a monoclonal antibody developed by AbCellera Biologics and Eli Lilly as a treatment for COVID-19. The medication was granted an emergency use authorization (EUA) by the US Food and Drug Administration (FDA) in November 2020, and the EUA was revoked in April 2021.
Bamlanivimab/etesevimab is a combination of two monoclonal antibodies, bamlanivimab and etesevimab, administered together via intravenous infusion as a treatment for COVID-19. Both types of antibody target the surface spike protein of SARS‑CoV‑2.
Frunevetmab, sold under the brand name Solensia, is a monoclonal antibody used to treat pain associated with osteoarthritis in cats. It is the first monoclonal antibody drug approved by the US Food and Drug Administration for animal use. Frunevetmab is the international nonproprietary name.
Teclistamab, sold under the brand name Tecvayli, is a human bispecific monoclonal antibody used for the treatment of relapsed and refractory multiple myeloma. It is a bispecific antibody that targets the CD3 receptor expressed on the surface of T-cells and B-cell maturation antigen (BCMA), which is expressed on the surface of malignant multiple myeloma B-lineage cells.
References
- ↑ "LIBRELA Product information". Health Canada.
- ↑ "Health product highlights 2021: Annexes of products approved in 2021". Health Canada . 3 August 2022. Retrieved 25 March 2024.
- 1 2 "Librela (bedinvetmab injection) Injectable Solution Dogs". Zoetis Inc. U.S. Food and Drug Administration. NADA 141-562.
- 1 2 3 4 "Librela EPAR". European Medicines Agency (EMA). 21 February 2022. Archived from the original on 16 March 2023. Retrieved 13 May 2023.
- 1 2 3 "Zoetis Announces U.S. FDA Approval of Librela (bedinvetmab injection) to Control Osteoarthritis (OA) Pain in Dogs" (Press release). Zoetis. 5 May 2023. Archived from the original on 6 May 2023. Retrieved 13 May 2023– via Business Wire.
- 1 2 3 4 5 6 7 8 9 10 11 "FDA Approves First Monoclonal Antibody for Dogs with Osteoarthritis Pain". U.S. Food and Drug Administration (FDA). 5 May 2023. Retrieved 13 May 2023.
 This article incorporates text from this source, which is in the public domain .
This article incorporates text from this source, which is in the public domain . - ↑ Krautmann M, Walters R, Cole P, Tena J, Bergeron LM, Messamore J, et al. (October 2021). "Laboratory safety evaluation of bedinvetmab, a canine anti-nerve growth factor monoclonal antibody, in dogs". Veterinary Journal. 276: 105733. doi: 10.1016/j.tvjl.2021.105733 . PMID 34391918.
- ↑ "US FDA CVM November 20, 2023 Untitled Letter to Zoetis re: NADA 141-562". US Food and Drug Administration.
- ↑ "US FDA CVM May 30, 2024 CVM FOIA Electronic Reading Room". US Food and Drug Administration.
- ↑ "EudraVigilance - European database of suspected adverse drug reaction reports". European Medicines Agency.
- ↑ World Health Organization (2019). "International nonproprietary names for pharmaceutical substances (INN): recommended INN: list 82". WHO Drug Information. 33 (3). hdl: 10665/330879 .