
In genetics and developmental biology, somatic cell nuclear transfer (SCNT) is a laboratory strategy for creating a viable embryo from a body cell and an egg cell. The technique consists of taking an enucleated oocyte and implanting a donor nucleus from a somatic (body) cell. It is used in both therapeutic and reproductive cloning. In 1996, Dolly the sheep became famous for being the first successful case of the reproductive cloning of a mammal. In January 2018, a team of scientists in Shanghai announced the successful cloning of two female crab-eating macaques from fetal nuclei.

Styrofoam is a trademarked brand of closed-cell extruded polystyrene foam (XPS), commonly called "Blue Board", manufactured as foam continuous building insulation board used in walls, roofs, and foundations as thermal insulation and water barrier. This material is light blue in color and is owned and manufactured by The Dow Chemical Company.
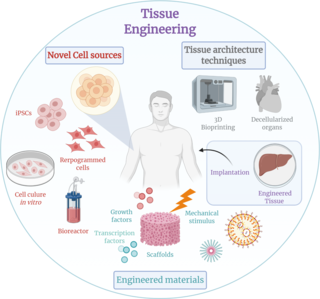
Tissue engineering is a biomedical engineering discipline that uses a combination of cells, engineering, materials methods, and suitable biochemical and physicochemical factors to restore, maintain, improve, or replace different types of biological tissues. Tissue engineering often involves the use of cells placed on tissue scaffolds in the formation of new viable tissue for a medical purpose but is not limited to applications involving cells and tissue scaffolds. While it was once categorized as a sub-field of biomaterials, having grown in scope and importance it can be considered as a field in its own.
In vitro toxicity testing is the scientific analysis of the effects of toxic chemical substances on cultured bacteria or mammalian cells. In vitro testing methods are employed primarily to identify potentially hazardous chemicals and/or to confirm the lack of certain toxic properties in the early stages of the development of potentially useful new substances such as therapeutic drugs, agricultural chemicals and food additives.

Cultured meat is meat produced by in vitro cell culture of animal cells, instead of from slaughtered animals. It is a form of cellular agriculture.

Embryonic stem cells are pluripotent stem cells derived from the inner cell mass of a blastocyst, an early-stage pre-implantation embryo. Human embryos reach the blastocyst stage 4–5 days post fertilization, at which time they consist of 50–150 cells. Isolating the embryoblast, or inner cell mass (ICM) results in destruction of the blastocyst, a process which raises ethical issues, including whether or not embryos at the pre-implantation stage have the same moral considerations as embryos in the post-implantation stage of development.
Fetal bovine serum (FBS) comes from the blood drawn from a bovine fetus via a closed system of collection at the slaughterhouse. Fetal bovine serum is the most widely used serum-supplement for the in vitro cell culture of eukaryotic cells. This is due to it having a very low level of antibodies and containing more growth factors, allowing for versatility in many different cell culture applications.
A biopharmaceutical, also known as a biologic(al) medical product, or biologic, is any pharmaceutical drug product manufactured in, extracted from, or semisynthesized from biological sources. Different from totally synthesized pharmaceuticals, they include vaccines, whole blood, blood components, allergenics, somatic cells, gene therapies, tissues, recombinant therapeutic protein, and living medicines used in cell therapy. Biologics can be composed of sugars, proteins, nucleic acids, or complex combinations of these substances, or may be living cells or tissues. They are isolated from living sources—human, animal, plant, fungal, or microbial. They can be used in both human and animal medicine.
MilliporeSigma is a German chemical, life science and biotechnology company, before 2014 known as Sigma-Aldrich, owned by Merck KGaA.
Stem cell genomics analyzes the genomes of stem cells. Currently, this field is rapidly expanding due to the dramatic decrease in the cost of sequencing genomes. The study of stem cell genomics has wide reaching implications in the study of stem cell biology and possible therapeutic usages of stem cells. Application of research in this field could lead to drug discovery and information on diseases by the molecular characterization of the pluripotent stem cell through DNA and transcriptome sequencing and looking at the epigenetic changes of stem cells and subsequent products. One step in that process is single cell phenotypic analysis, and the connection between the phenotype and genotype of specific stem cells. While current genomic screens are done with entire populations of cells, focusing in on a single stem cell will help determine specific signaling activity associated with varying degrees of stem cell differentiation and limit background due to heterogeneous populations. Single cell analysis of induced pluripotent stem cells (iPSCs), or stem cells able to differentiate into many different cell types, is a suggested method for treating such diseases like Alzheimer's disease (AD). This includes for understanding the differences between sporadic AD and familial AD. By first taking a skin sample from the patient and are transformed by transducing cells using retroviruses to encode such stem cell genes as Oct4, Sox2, KLF4 and cMYC. This allows for skin cells to be reprogrammed into patient-specific stem cell lines. Taking genomic sequences of these individual cells would allow for patient-specific treatments and furthering understanding of AD disease models. This technique would be used for similar diseases, like amyotrophic lateral sclerosis (ALS) and spinal muscular atrophy (SMA). These stem cells developed from a singular patient would also be able to be used to produce cells affected in the above-mentioned diseases. As mentioned, it will also lead to patient specific phenotypes of each disease. Further chemical analyses to develop safer drugs can be done through sequence information and cell-culture tests on iPSCs. After development on a specific drug, it can be transferred to other patient diseased cells while also being safety tested.

Cryo-preservation or cryo-conservation is a process where organelles, cells, tissues, extracellular matrix, organs, or any other biological constructs susceptible to damage caused by unregulated chemical kinetics are preserved by cooling to very low temperatures. At low enough temperatures, any enzymatic or chemical activity which might cause damage to the biological material in question is effectively stopped. Cryopreservation methods seek to reach low temperatures without causing additional damage caused by the formation of ice crystals during freezing. Traditional cryopreservation has relied on coating the material to be frozen with a class of molecules termed cryoprotectants. New methods are being investigated due to the inherent toxicity of many cryoprotectants. Cryoconservation of animal genetic resources is done with the intention of conservation of the breed.
Stem cell laws are the law rules, and policy governance concerning the sources, research, and uses in treatment of stem cells in humans. These laws have been the source of much controversy and vary significantly by country. In the European Union, stem cell research using the human embryo is permitted in Sweden, Spain, Finland, Belgium, Greece, Britain, Denmark and the Netherlands; however, it is illegal in Canada, Germany, Austria, Ireland, Italy, and Portugal. The issue has similarly divided the United States, with several states enforcing a complete ban and others giving support. Elsewhere, Japan, India, Iran, Israel, South Korea, China, and Australia are supportive. However, New Zealand, most of Africa, and most of South America are restrictive.
Human platelet lysate is a substitute supplement for fetal bovine serum (FBS) in experimental and clinical cell culture. It is a turbid, light-yellow liquid that is obtained from human blood platelets after freeze/thaw cycle(s). The freeze/thaw cycle causes the platelets to lyse, releasing a large quantity of growth factors necessary for cell expansion. FBS-free cell culture media, e.g. with platelet lysate or chemically defined/ animal component free, are used for cell therapy or regenerative medicine. They are commercially available in GMP -quality which is generally basis for regulatory approval.

George Quentin Daley is the Dean of the Faculty of Medicine, Caroline Shields Walker Professor of Medicine, and Professor of Biological Chemistry and Molecular Pharmacology at Harvard Medical School. He was formerly the Robert A. Stranahan Professor of Pediatrics at Harvard Medical School, Director of the Stem Cell Transplantation Program at Boston Children's Hospital, and an investigator of the Howard Hughes Medical Institute, Associate Director of Children’s Stem Cell Program, a member of the Executive Committee of the Harvard Stem Cell Institute. He is a past president of the International Society for Stem Cell Research (2007–2008).
Stemgent is an American privately funded biotech company focused on providing reagents and technology developed by some of the world's leading stem cell scientists. Founded in 2008, Stemgent has two fully operational facilities in both San Diego, California and Cambridge, Massachusetts. Stemgent currently has 40 employees. The company is designed to serve researchers who study stem cell biology and regenerative medicine and those who use cells derived from stem cells as tools to advance their understanding of major diseases.
A chemically defined medium is a growth medium suitable for the in vitro cell culture of human or animal cells in which all of the chemical components are known. Standard cell culture media commonly consist of a basal medium supplemented with animal serum as a source of nutrients and other ill-defined factors. The technical disadvantages to using serum include its undefined nature, batch-to-batch variability in composition, and the risk of contamination.
A cell bank is a facility that stores cells of specific genome for the purpose of future use in a product or medicinal needs. They often contain expansive amounts of base cell material that can be utilized for various projects. Cell banks can be used to generate detailed characterizations of cell lines and can also help mitigate cross-contamination of a cell line. Utilizing cell banks also reduces the cost of cell culture processes, providing a cost-efficient alternative to keeping cells in culture constantly. Cell banks are commonly used within fields including stem cell research and pharmaceuticals, with cryopreservation being the traditional method of keeping cellular material intact. Cell banks also effectively reduce the frequency of a cell sample diversifying from natural cell divisions over time.
STEMCELL Technologies Inc. is a global biotechnology company that develops, manufactures and sells products and provides services that support academic and industrial scientists. The company specializes in developing cell culture media, cell separation products, instruments and other reagents for use in stem cell, immunology, cancer, regenerative medicine and cellular therapy research. STEMCELL's Research and Development team often collaborates with academic and industrial partners to develop, produce and distribute products specific to a given research field. STEMCELL has helped several scientific technologies born in academic research settings to reach the global biotechnology market. STEMCELL is headquartered in Vancouver, British Columbia, Canada, and has offices in eight countries including the United States, United Kingdom, Germany, France, Australia, Singapore and China, as well as distributors in approximately 80 other countries. STEMCELL is the largest biotechnology company in Canada, currently employing more than 1400 people globally and offers a catalogue of more than 2,200 products.

Fujifilm Cellular Dynamics, Inc. (FCDI) is a large scale manufacturer of human cells, created from induced pluripotent stem cells, for use in basic research, drug discovery and regenerative medicine applications.
Cellular agriculture focuses on the production of agriculture products from cell cultures using a combination of biotechnology, tissue engineering, molecular biology, and synthetic biology to create and design new methods of producing proteins, fats, and tissues that would otherwise come from traditional agriculture. Most of the industry is focused on animal products such as meat, milk, and eggs, produced in cell culture rather than raising and slaughtering farmed livestock. The most well known cellular agriculture concept is cultured meat.







