
An ideal gas is a theoretical gas composed of many randomly moving point particles that are not subject to interparticle interactions. The ideal gas concept is useful because it obeys the ideal gas law, a simplified equation of state, and is amenable to analysis under statistical mechanics. The requirement of zero interaction can often be relaxed if, for example, the interaction is perfectly elastic or regarded as point-like collisions.
In mathematics, the Laplace operator or Laplacian is a differential operator given by the divergence of the gradient of a function on Euclidean space. It is usually denoted by the symbols , , or . In a Cartesian coordinate system, the Laplacian is given by the sum of second partial derivatives of the function with respect to each independent variable. In other coordinate systems, such as cylindrical and spherical coordinates, the Laplacian also has a useful form. Informally, the Laplacian Δf(p) of a function f at a point p measures by how much the average value of f over small spheres or balls centered at p deviates from f(p).
The Gibbs–Helmholtz equation is a thermodynamic equation used for calculating changes in the Gibbs energy of a system as a function of temperature. It is named after Josiah Willard Gibbs and Hermann von Helmholtz.

In thermodynamics, the Gibbs free energy is a thermodynamic potential that can be used to calculate the maximum reversible work that may be performed by a thermodynamic system at a constant temperature and pressure. The Gibbs free energy (, measured in joules in SI) is the maximum amount of non-expansion work that can be extracted from a thermodynamically closed system. This maximum can be attained only in a completely reversible process. When a system transforms reversibly from an initial state to a final state, the decrease in Gibbs free energy equals the work done by the system to its surroundings, minus the work of the pressure forces.

A thermodynamic potential is a scalar quantity used to represent the thermodynamic state of a system. The concept of thermodynamic potentials was introduced by Pierre Duhem in 1886. Josiah Willard Gibbs in his papers used the term fundamental functions. One main thermodynamic potential that has a physical interpretation is the internal energy U. It is the energy of configuration of a given system of conservative forces and only has meaning with respect to a defined set of references. Expressions for all other thermodynamic energy potentials are derivable via Legendre transforms from an expression for U. In thermodynamics, external forces, such as gravity, are typically disregarded when formulating expressions for potentials. For example, while all the working fluid in a steam engine may have higher energy due to gravity while sitting on top of Mount Everest than it would at the bottom of the Mariana Trench, the gravitational potential energy term in the formula for the internal energy would usually be ignored because changes in gravitational potential within the engine during operation would be negligible. In a large system under even homogeneous external force, like the earth atmosphere under gravity, the intensive parameters should be studied locally having even in equilibrium different values in different places far from each other.

In thermodynamics, the Helmholtz free energy is a thermodynamic potential that measures the useful work obtainable from a closed thermodynamic system at a constant temperature (isothermal). The change in the Helmholtz energy during a process is equal to the maximum amount of work that the system can perform in a thermodynamic process in which temperature is held constant. At constant temperature, the Helmholtz free energy is minimized at equilibrium.

The internal energy of a thermodynamic system is the energy contained within it. It is the energy necessary to create or prepare the system in any given internal state. It does not include the kinetic energy of motion of the system as a whole, nor the potential energy of the system as a whole due to external force fields, including the energy of displacement of the surroundings of the system. It keeps account of the gains and losses of energy of the system that are due to changes in its internal state. The internal energy is measured as a difference from a reference zero defined by a standard state. The difference is determined by thermodynamic processes that carry the system between the reference state and the current state of interest.

In mathematics and physics, the Legendre transformation, named after Adrien-Marie Legendre, is an involutive transformation on the real-valued convex functions of one real variable. In physical problems, it is used to convert functions of one quantity into functions of the conjugate quantity. In this way, it is commonly used in classical mechanics to derive the Hamiltonian formalism out of the Lagrangian formalism and in thermodynamics to derive the thermodynamic potentials, as well as in the solution of differential equations of several variables.

In fluid dynamics, the Euler equations are a set of quasilinear hyperbolic equations governing adiabatic and inviscid flow. They are named after Leonhard Euler. The equations represent Cauchy equations of conservation of mass (continuity), and balance of momentum and energy, and can be seen as particular Navier–Stokes equations with zero viscosity and zero thermal conductivity. In fact, Euler equations can be obtained by linearization of some more precise continuity equations like Navier–Stokes equations in a local equilibrium state given by a Maxwellian. The Euler equations can be applied to incompressible and to compressible flow – assuming the flow velocity is a solenoidal field, or using another appropriate energy equation respectively. Historically, only the incompressible equations have been derived by Euler. However, fluid dynamics literature often refers to the full set – including the energy equation – of the more general compressible equations together as "the Euler equations".
In multivariate calculus, a differential is said to be exact or perfect, as contrasted with an inexact differential, if it is of the form dQ, for some differentiable function Q.
In chemistry, an ideal solution or ideal mixture is a solution in which the gas phase exhibits thermodynamic properties analogous to those of a mixture of ideal gases. The enthalpy of mixing is zero as is the volume change on mixing by definition; the closer to zero the enthalpy of mixing is, the more "ideal" the behaviour of the solution becomes. The vapor pressure of the solution obeys either Raoult's law or Henry's law, and the activity coefficient of each component is equal to one.
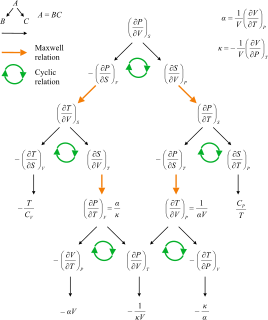
Maxwell's relations are a set of equations in thermodynamics which are derivable from the symmetry of second derivatives and from the definitions of the thermodynamic potentials. These relations are named for the nineteenth-century physicist James Clerk Maxwell.
In chemical thermodynamics, the fugacity of a real gas is an effective partial pressure which replaces the mechanical partial pressure in an accurate computation of the chemical equilibrium constant. It is equal to the pressure of an ideal gas which has the same temperature and molar Gibbs free energy as the real gas.

Thermodynamics is expressed by a mathematical framework of thermodynamic equations which relate various thermodynamic quantities and physical properties measured in a laboratory or production process. Thermodynamics is based on a fundamental set of postulates, that became the laws of thermodynamics.

The thermodynamic properties of materials are intensive thermodynamic parameters which are specific to a given material. Each is directly related to a second order differential of a thermodynamic potential. Examples for a simple 1-component system are:

A thermodynamic free entropy is an entropic thermodynamic potential analogous to the free energy. Also known as a Massieu, Planck, or Massieu–Planck potentials, or (rarely) free information. In statistical mechanics, free entropies frequently appear as the logarithm of a partition function. The Onsager reciprocal relations in particular, are developed in terms of entropic potentials. In mathematics, free entropy means something quite different: it is a generalization of entropy defined in the subject of free probability.

In thermodynamics, the fundamental thermodynamic relation are four fundamental equations which demonstrate how four important thermodynamic quantities depend on variables that can be controlled and measured experimentally. Thus, they are essentially equations of state, and using the fundamental equations, experimental data can be used to determine sought-after quantities like G or H. The relation is generally expressed as a microscopic change in internal energy in terms of microscopic changes in entropy, and volume for a closed system in thermal equilibrium in the following way.

This article is a summary of common equations and quantities in thermodynamics.
In astrophysics, what is referred to as "entropy" is actually the adiabatic constant derived as follows.

The thermodynamic square is a mnemonic diagram attributed to Max Born and used to help determine thermodynamic relations. Born presented the thermodynamic square in a 1929 lecture. The symmetry of thermodynamics appears in a paper by F.O. Koenig. The corners represent common conjugate variables while the sides represent thermodynamic potentials. The placement and relation among the variables serves as a key to recall the relations they constitute.























































