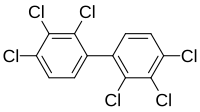
Polychlorinated biphenyls (PCBs) are highly carcinogenic chemical compounds, formerly used in industrial and consumer products, whose production was banned in the United States by the Toxic Substances Control Act in 1976 and internationally by the Stockholm Convention on Persistent Organic Pollutants in 2001.
Polychlorinated dibenzodioxins (PCDDs), or simply dioxins, are a group of long-lived polyhalogenated organic compounds that are primarily anthropogenic, and contribute toxic, persistent organic pollution in the environment.

Chloracne is an acneiform eruption of blackheads, cysts, and pustules associated with exposure to certain halogenated aromatic compounds, such as chlorinated dioxins and dibenzofurans. The lesions are most frequently found on the cheeks, behind the ears, in the armpits and groin region.
Polybrominated diphenyl ethers or PBDEs, are a class of organobromine compounds that are used as flame retardants. Like other brominated flame retardants, PBDEs have been used in a wide array of products, including building materials, electronics, furnishings, motor vehicles, airplanes, plastics, polyurethane foams, and textiles. They are structurally akin to polychlorinated diphenyl ethers (PCDEs), polychlorinated biphenyls (PCBs) and other polyhalogenated compounds, consisting of two halogenated aromatic rings. PBDEs are classified according to the average number of bromine atoms in the molecule. The life-saving benefits of fire retardants led to their popularization. Standards for mass transit vehicles continues to increase as of 2021.
Halocarbon compounds are chemical compounds in which one or more carbon atoms are linked by covalent bonds with one or more halogen atoms resulting in the formation of organofluorine compounds, organochlorine compounds, organobromine compounds, and organoiodine compounds. Chlorine halocarbons are the most common and are called organochlorides.

Dibenzofuran is a heterocyclic organic compound with the chemical structure shown at right. It is an aromatic compound that has two benzene rings fused to a central furan ring. All the numbered carbon atoms have a hydrogen atom bonded to each of them. It is a volatile white solid that is soluble in nonpolar organic solvents. It is obtained from coal tar, where it exists as a 1% component.

Persistent organic pollutants (POPs) are organic compounds that are resistant to degradation through chemical, biological, and photolytic processes. They are toxic and adversely affect human health and the environment around the world. Because they can be transported by wind and water, most POPs generated in one country can and do affect people and wildlife far from where they are used and released.

Polybrominated biphenyls (PBBs), also called brominated biphenyls or polybromobiphenyls, are a group of manufactured chemicals that consist of polyhalogenated derivatives of a biphenyl core. Their chlorine analogs are the PCBs. While once widely used commercially, PBBs are now controlled substances under the Restriction of Hazardous Substances Directive, which limits their use in electrical and electronic products sold in the EU.
Transformer oil or insulating oil is an oil that is stable at high temperatures and has excellent electrical insulating properties. It is used in oil-filled wet transformers, some types of high-voltage capacitors, fluorescent lamp ballasts, and some types of high-voltage switches and circuit breakers. Its functions are to insulate, suppress corona discharge and arcing, and to serve as a coolant.

Yushō disease was a mass poisoning by polychlorinated biphenyls (PCBs) which occurred in northern Kyūshū, Japan, in 1968. In January 1968, rice bran oil produced by Kanemi Company in Kyushu was contaminated with PCBs and polychlorinated dibenzofurans (PCDFs) during production. For deodorization, the oil was heated using PCB as the heating medium, circulating through pipes. Due to holes in the pipes the PCB leaked into the rice bran oil. The contaminated rice bran oil was then sold to poultry farmers for use as a feed supplement and to consumers for use in cooking. In February to March 1968, farmers started reporting that their poultry were dying due to apparent difficulty in breathing; altogether 400,000 birds died. About 14,000 people who had consumed the contaminated rice oil were affected in Japan. More than 500 died. Common symptoms included dermal and ocular lesions, and a lowered immune response. Other symptoms included fatigue, headache, cough, and unusual skin sores. Additionally, in children, there were reports of poor cognitive development.

Polychlorinated dibenzofurans (PCDFs) are a family of organic compounds with one or several of the hydrogens in the dibenzofuran structure replaced by chlorines. For example, 2,3,7,8-tetrachlorodibenzofuran (TCDF) has chlorine atoms substituted for each of the hydrogens on the number 2, 3, 7, and 8 carbons. Polychlorinated dibenzofurans with chlorines at least in positions 2,3,7 and 8 are much more toxic than the parent compound dibenzofurane, with properties and chemical structures similar to polychlorinated dibenzodioxins. These groups together are often inaccurately called dioxins. They are known developmental toxicants, and suspected human carcinogens. PCDFs tend to co-occur with polychlorinated dibenzodioxins (PCDDs). PCDFs can be formed by pyrolysis or incineration at temperatures below 1200 °C of chlorine containing products, such as PVC, PCBs, and other organochlorides, or of non-chlorine containing products in the presence of chlorine donors. Dibenzofurans are known persistent organic pollutants (POP), classified among the dirty dozen in the Stockholm Convention on Persistent Organic Pollutants.

Dioxins and dioxin-like compounds (DLCs) are a group of chemical compounds that are persistent organic pollutants (POPs) in the environment. They are mostly by-products of burning or various industrial processes or, in the case of dioxin-like PCBs and PBBs, unwanted minor components of intentionally produced mixtures.
A polyhalogenated compound (PHC) is any compound with multiple substitutions of halogens. They are of particular interest and importance because they bioaccumulate in humans, and comprise a superset of which has many toxic and carcinogenic industrial chemicals as members. PBDEs, PCBs, dioxins (PCDDs) and PFCs are all polyhalogenated compounds. They are generally non-miscible in organic solvents or water, but miscible in some hydrocarbons from which they often derive.
Chlorinated polycyclic aromatic hydrocarbons (Cl-PAHs) are a group of compounds comprising polycyclic aromatic hydrocarbons with two or more aromatic rings and one or more chlorine atoms attached to the ring system. Cl-PAHs can be divided into two groups: chloro-substituted PAHs, which have one or more hydrogen atoms substituted by a chlorine atom, and chloro-added Cl-PAHs, which have two or more chlorine atoms added to the molecule. They are products of incomplete combustion of organic materials. They have many congeners, and the occurrences and toxicities of the congeners differ. Cl-PAHs are hydrophobic compounds and their persistence within ecosystems is due to their low water solubility. They are structurally similar to other halogenated hydrocarbons such as polychlorinated dibenzo-p-dioxins (PCDDs), dibenzofurans (PCDFs), and polychlorinated biphenyls (PCBs). Cl-PAHs in the environment are strongly susceptible to the effects of gas/particle partitioning, seasonal sources, and climatic conditions.

2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) is a polychlorinated dibenzo-p-dioxin (sometimes shortened, though inaccurately, to simply 'dioxin') with the chemical formula C12H4Cl4O2. Pure TCDD is a colorless solid with no distinguishable odor at room temperature. It is usually formed as an unwanted product in burning processes of organic materials or as a side product in organic synthesis.
Toxic equivalency factor (TEF) expresses the toxicity of dioxins, furans and PCBs in terms of the most toxic form of dioxin, 2,3,7,8-TCDD. The toxicity of the individual congeners may vary by orders of magnitude.
Persistent, bioaccumulative and toxic substances (PBTs) are a class of compounds that have high resistance to degradation from abiotic and biotic factors, high mobility in the environment and high toxicity. Because of these factors PBTs have been observed to have a high order of bioaccumulation and biomagnification, very long retention times in various media, and widespread distribution across the globe. Most PBTs in the environment are either created through industry or are unintentional byproducts.
Toxicodynamics, termed pharmacodynamics in pharmacology, describes the dynamic interactions of a toxicant with a biological target and its biological effects. A biological target, also known as the site of action, can be binding proteins, ion channels, DNA, or a variety of other receptors. When a toxicant enters an organism, it can interact with these receptors and produce structural or functional alterations. The mechanism of action of the toxicant, as determined by a toxicant’s chemical properties, will determine what receptors are targeted and the overall toxic effect at the cellular level and organismal level.

1,2,3,4,6,7,8-Heptachlorodibenzo-para-dioxin (often referred to as 1,2,3,4,6,7,8-HpCDD) is a polychlorinated derivative of dibenzo-p-dioxin and can therefore be categorized as polychlorinated dibenzo-p-dioxin (PCDD), a subclass of dioxins which includes 75 congeners. HpCDD is the dibenzo-p-dioxin which is chlorinated at positions 1, 2, 3, 4, 6, 7, and 8. It is a polycyclic heterocyclic organic compound, since HpCDD contains multiple cyclic structures (two benzene rings connected by a 1,4-dioxin ring) in which two different elements (carbon and oxygen) are members of its rings. HpCDD has molecular formula C12HCl7O2 and is an off-white powder, which is insoluble in water.

Deborah Liebl Swackhamer was an environmental chemist and professor emerita at the University of Minnesota in Minneapolis. Swackhamer applied her expertise in studying the effects of exposure to toxic chemicals, as well as the processes that spread those chemicals, to developing policies that address exposure risks.