Bioorganic chemistry is a scientific discipline that combines organic chemistry and biochemistry. It is that branch of life science that deals with the study of biological processes using chemical methods. Protein and enzyme function are examples of these processes.

(+)-CPCA is a stimulant drug similar in structure to pethidine and to RTI-31, but nocaine is lacking the two-carbon bridge of RTI-31's tropane skeleton. This compound was first developed as a substitute agent for cocaine.

The adenosine A2B receptor, also known as ADORA2B, is a G-protein coupled adenosine receptor, and also denotes the human adenosine A2b receptor gene which encodes it.

Fuculose or 6-deoxy-tagatose is a ketohexose deoxy sugar. Fuculose is involved in the process of sugar metabolism. l-Fuculose can be formed from l-fucose by l-fucose isomerase and converted to L-fuculose-1-phosphate by l-fuculose kinase.
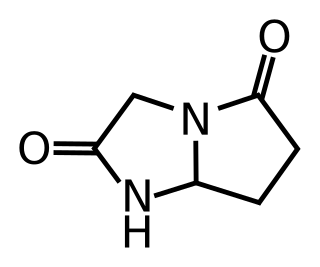
Dimiracetam is a nootropic drug of the racetam family, derivatives of which may have application in the treatment of neuropathic pain.

Efaroxan is an α2-adrenergic receptor antagonist and antagonist of the imidazoline receptor.
Ibipinabant (SLV319, BMS-646,256) is a drug used in scientific research which acts as a potent and highly selective CB1 antagonist. It has potent anorectic effects in animals, and was researched for the treatment of obesity, although CB1 antagonists as a class have now fallen out of favour as potential anorectics following the problems seen with rimonabant, and so ibipinabant is now only used for laboratory research, especially structure-activity relationship studies into novel CB1 antagonists. SLV330, which is a structural analogue of Ibipinabant, was reported active in animal models related to the regulation of memory, cognition, as well as in addictive behavior. An atom-efficient synthesis of ibipinabant has been reported.

AMDA (9-Aminomethyl-9,10-dihydroanthracene) is an organic compound which acts as a potent and selective antagonist for the 5-HT2A receptor. It has been used to help study the shape of the 5-HT2A protein, and develop a large family of related derivatives with even higher potency and selectivity.

Bioorganic & Medicinal Chemistry Letters is a scientific journal focusing on the results of research on the molecular structure of biological organisms and the interaction of biological targets with chemical agents. It is published by Elsevier, which also publishes Bioorganic & Medicinal Chemistry for longer works.
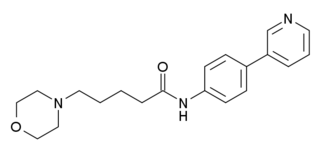
WAY-317538 (SEN-12333) is a drug that acts as a potent and selective full agonist for the α7 subtype of neural nicotinic acetylcholine receptors. It was not the most potent compound in the series, but was selected for further development on the basis of its high selectivity over related receptors, ease of synthesis, and good in vivo properties including high oral bioavailability and good brain penetration. It has nootropic and neuroprotective effects in animal studies, and is being investigated as a potential treatment for neurodegenerative and neurocognitive conditions including Alzheimer's disease and schizophrenia.
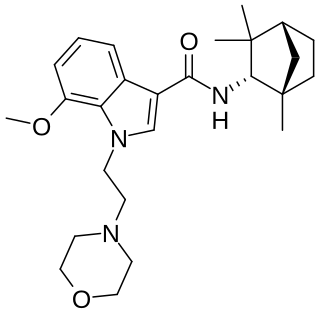
MN-25 (UR-12) is a drug invented by Bristol-Myers Squibb, that acts as a reasonably selective agonist of peripheral cannabinoid receptors. It has moderate affinity for CB2 receptors with a Ki of 11 nM, but 22x lower affinity for the psychoactive CB1 receptors with a Ki of 245 nM. The indole 2-methyl derivative has the ratio of affinities reversed however, with a Ki of 8 nM at CB1 and 29 nM at CB2, which contrasts with the usual trend of 2-methyl derivatives having increased selectivity for CB2 (cf. JWH-018 vs JWH-007, JWH-081 vs JWH-098).

Substituted tryptamines, or serotonin analogues, are organic compounds which may be thought of as being derived from tryptamine itself. The molecular structures of all tryptamines contain an indole ring, joined to an amino (NH2) group via an ethyl (−CH2–CH2−) sidechain. In substituted tryptamines, the indole ring, sidechain, and/or amino group are modified by substituting another group for one of the hydrogen (H) atoms.

Biphalin is a dimeric enkephalin endogenous peptide (Tyr-D-Ala-Gly-Phe-NH)2 composed of two tetrapeptides derived from enkephalins, connected 'tail-to-tail' by a hydrazide bridge. The presence of two distinct pharmacophores confers on biphalin a high affinity for both μ and δ opioid receptors (with an EC50 of about 1-5 nM for both μ and δ receptors), therefore it has analgesic activity. Biphalin presents a considerable antinociceptive profile. In fact, when administered intracerebroventricularly in mice, biphalin displays a potency almost 7-fold greater than that of the ultra-potent alkaloid agonist, etorphine and 7000-fold greater than morphine; biphalin and morphine were found to be equipotent after intraperitoneal administration. The extraordinary in vivo potency shown by this compound is coupled with low side-effects, in particular, to produce no dependency in chronic use. For these reasons, several efforts have been carried out in order to obtain more information about structure-activity relationship (SAR). Results clearly indicate that, at least for μ receptor binding, the presence of two pharmacophores is not necessary; Tyr1 is indispensable for analgesic activity, while replacing Phe at the position 4 and 4' with non-aromatic, but lipophilic amino acids does not greatly change the binding properties and in general 4,4' positions are found to be important to design biphalin analogues with increased potency and modified μ/δ selectivity. The hydrazide linker is not fundamental for activity or binding, and it can be conveniently substituted by different conformationally constrained cycloaliphatic diamine linkers.

KM-233 is a synthetic cannabinoid drug which is a structural analog of Δ8-tetrahydrocannabinol (THC), the less active but more stable isomer of the active component of Cannabis. KM-233 differs from Δ8-THC by the pentyl side chain being replaced by a 1,1-dimethylbenzyl group. It has high binding affinity in vitro for both the CB1 and CB2 receptors, with a CB2 affinity of 0.91 nM and 13-fold selectivity over the CB1 receptor. In animal studies, it has been found to be a potential treatment for glioma, a form of brain tumor. Many related analogues are known where the 1,1-dimethylbenzyl group is substituted or replaced by other groups, with a fairly well established structure-activity relationship.

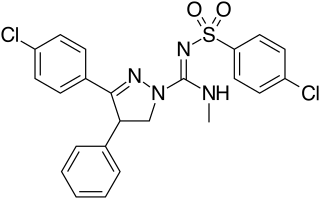
QMPSB is an arylsulfonamide-based synthetic cannabinoid that has been sold as a designer drug.

NC 45-0095 is a synthetic nonsteroidal selective estrogen receptor modulator (SERM) which was under development by Novo Nordisk for the treatment of postmenopausal osteoporosis but was never marketed. It is a partial agonist of the estrogen receptor (IC50 (for binding inhibition) = 9.5 nM; EC50 = 13 nM) with mixed estrogenic and antiestrogenic activity, and shows full estrogenic activity in bone and uterus (Emax (relative to moxestrol, in Ishikawa endometrial cancer cell line) = 105%). The compound is a pyrroloindolizine derivative. Its development was discontinued by 2003.

Mozenavir (DMP-450) is an antiviral drug which was developed as a treatment for HIV/AIDS. It acts as an HIV protease inhibitor and binds to this target with high affinity, however despite promising results in early testing, mozenavir was unsuccessful in human clinical trials. Studies continue into related derivatives.
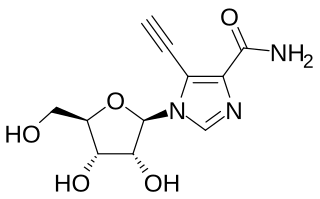
EICAR is a drug which acts as an inhibitor of the enzyme IMP dehydrogenase. It is a nucleoside derivative which has both anti-cancer and antiviral effects, and was originally developed for the treatment of leukemia, but was unsuccessful in human clinical trials. It has broad spectrum antiviral effects with activity against pox viruses, Semliki forest virus, Junin virus, reovirus, influenza, measles virus and respiratory syncytial virus among others, although it is not active against coronaviridae such as SARS-CoV-1. This useful spectrum of activity means that EICAR and related derivatives continue to be investigated for the treatment of viral diseases.
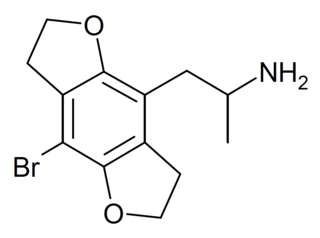
DOB-FLY is a recreational designer drug with psychedelic effects. It can be regarded as the alpha-methyl derivative of 2C-B-FLY or the partially saturated counterpart of bromo-dragonfly. Unlike bromo-dragonfly, DOB-FLY is only slightly more potent than DOB itself, with an active dose in humans of around 1 mg.


















