
Brownian motion, or pedesis, is the random motion of particles suspended in a medium.

In cell biology, the cytoplasm is all of the material within a eukaryotic cell, enclosed by the cell membrane, except for the cell nucleus. The material inside the nucleus and contained within the nuclear membrane is termed the nucleoplasm. The main components of the cytoplasm are cytosol, the organelles, and various cytoplasmic inclusions. The cytoplasm is about 80% water and usually colorless.

A colloid is a mixture in which one substance of microscopically dispersed insoluble particles are suspended throughout another substance. However, some definitions specify that the particles must be dispersed in a liquid, and others extend the definition to include substances like aerosols and gels. The term colloidal suspension refers unambiguously to the overall mixture. A colloid has a dispersed phase and a continuous phase. The dispersed phase particles have a diameter of approximately 1 nanometre to 1 micrometre.

Brownian motors are nanoscale or molecular machines that use chemical reactions to generate directed motion in space. The theory behind Brownian motors relies on the phenomena of Brownian motion, random motion of particles suspended in a fluid resulting from their collision with the fast-moving molecules in the fluid.
Sedimentation equilibrium in a suspension of different particles, such as molecules, exists when the rate of transport of each material in any one direction due to sedimentation equals the rate of transport in the opposite direction due to diffusion. Sedimentation is due to an external force, such as gravity or centrifugal force in a centrifuge.
A sol is a colloid made out of solid particles in a continuous liquid medium. Sols are quite stable and show the Tyndall effect. Examples include blood, pigmented ink, cell fluids, paint, antacids and mud.

Diffusion-limited aggregation (DLA) is the process whereby particles undergoing a random walk due to Brownian motion cluster together to form aggregates of such particles. This theory, proposed by T.A. Witten Jr. and L.M. Sander in 1981, is applicable to aggregation in any system where diffusion is the primary means of transport in the system. DLA can be observed in many systems such as electrodeposition, Hele-Shaw flow, mineral deposits, and dielectric breakdown.
In probability theory and statistics, a diffusion process is a solution to a stochastic differential equation. It is a continuous-time Markov process with almost surely continuous sample paths. Brownian motion, reflected Brownian motion and Ornstein–Uhlenbeck processes are examples of diffusion processes.
In materials science, the sol–gel process is a method for producing solid materials from small molecules. The method is used for the fabrication of metal oxides, especially the oxides of silicon (Si) and titanium (Ti). The process involves conversion of monomers into a colloidal solution (sol) that acts as the precursor for an integrated network of either discrete particles or network polymers. Typical precursors are metal alkoxides.
"Über die von der molekularkinetischen Theorie der Wärme geforderte Bewegung von in ruhenden Flüssigkeiten suspendierten Teilchen" is the 1905 journal article, by Albert Einstein, that proved the reality of atoms, which were first proposed in 1808 by John Dalton. It is one of the four groundbreaking papers Einstein published in 1905, in Annalen der Physik, in his miracle year.

Dynamic light scattering (DLS) is a technique in physics that can be used to determine the size distribution profile of small particles in suspension or polymers in solution. In the scope of DLS, temporal fluctuations are usually analyzed by means of the intensity or photon auto-correlation function. In the time domain analysis, the autocorrelation function (ACF) usually decays starting from zero delay time, and faster dynamics due to smaller particles lead to faster decorrelation of scattered intensity trace. It has been shown that the intensity ACF is the Fourier transformation of the power spectrum, and therefore the DLS measurements can be equally well performed in the spectral domain. DLS can also be used to probe the behavior of complex fluids such as concentrated polymer solutions.
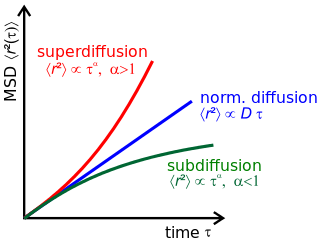
Anomalous diffusion is a diffusion process with a non-linear relationship between the mean squared displacement (MSD), , and time. This behavior is in stark contrast to Brownian motion, the typical diffusion process described by Einstein and Smoluchowski, where the MSD is a linear in time. Examples of anomalous diffusion in nature have been observed in biology in the cell nucleus, plasma membrane and cytoplasm.

Diffusiophoresis is the spontaneous motion of colloidal particles or molecules in a fluid, induced by a concentration gradient of a different substance. In other words, it is motion of one species, A, in response to a concentration gradient in another species, B. Typically, A is colloidal particles which are in aqueous solution in which B is a dissolved salt such as sodium chloride, and so the particles of A are much larger than the ions of B. But both A and B could be polymer molecules, and B could be a small molecule. For example, concentration gradients in ethanol solutions in water move 1 μm diameter colloidal particles with diffusiophoretic velocities of order 0.1 to 1 μm/s, the movement is towards regions of the solution with lower ethanol concentration. Both species A and B will typically be diffusing but diffusiophoresis is distinct from simple diffusion: in simple diffusion a species A moves down a gradient in its own concentration.

Particle size is a notion introduced for comparing dimensions of solid particles (flecks), liquid particles (droplets), or gaseous particles (bubbles). The notion of particle size applies to colloidal particles, particles in ecology, particles present in granular material, and particles that form a granular material.

In the mathematical field of probability, the Wiener sausage is a neighborhood of the trace of a Brownian motion up to a time t, given by taking all points within a fixed distance of Brownian motion. It can be visualized as a sausage of fixed radius whose centerline is Brownian motion. The Wiener sausage was named after Norbert Wiener by M. D. Donsker and S. R. Srinivasa Varadhan (1975) because of its relation to the Wiener process; the name is also a pun on Vienna sausage, as "Wiener" is German for "Viennese".

Diffusion is the net movement of anything from a region of higher concentration to a region of lower concentration. Diffusion is driven by a gradient in concentration.

A colloidal crystal is an ordered array of colloid particles and fine grained materials analogous to a standard crystal whose repeating subunits are atoms or molecules. A natural example of this phenomenon can be found in the gem opal, where spheres of silica assume a close-packed locally periodic structure under moderate compression. Bulk properties of a colloidal crystal depend on composition, particle size, packing arrangement, and degree of regularity. Applications include photonics, materials processing, and the study of self-assembly and phase transitions.

In the physical sciences, a particle is a small localized object to which can be ascribed several physical or chemical properties, such as volume, density, or mass. They vary greatly in size or quantity, from subatomic particles like the electron, to microscopic particles like atoms and molecules, to macroscopic particles like powders and other granular materials. Particles can also be used to create scientific models of even larger objects depending on their density, such as humans moving in a crowd or celestial bodies in motion.
Differential dynamic microscopy (DDM) is an optical technique that allows performing light scattering experiments by means of a simple optical microscope. DDM is suitable for typical soft materials such as for instance liquids or gels made of colloids, polymers and liquid crystals but also for biological materials like bacteria and cells.
In probability theory, the Brownian web is an uncountable collection of one-dimensional coalescing Brownian motions, starting from every point in space and time. It arises as the diffusive space-time scaling limit of a collection of coalescing random walks, with one walk starting from each point of the integer lattice Z at each time.












