Inbred strains are individuals of a particular species which are nearly identical to each other in genotype due to long inbreeding. A strain is inbred when it has undergone at least 20 generations of brother x sister or offspring x parent mating, at which point at least 98.6% of the loci in an individual of the strain will be homozygous, and each individual can be treated effectively as clones. Some inbred strains have been bred for over 150 generations, leaving individuals in the population to be isogenic in nature. Inbred strains of animals are frequently used in laboratories for experiments where for the reproducibility of conclusions all the test animals should be as similar as possible. However, for some experiments, genetic diversity in the test population may be desired. Thus outbred strains of most laboratory animals are also available, where an outbred strain is a strain of an organism that is effectively wildtype in nature, where there is as little inbreeding as possible.

Regulation of gene expression, or gene regulation, includes a wide range of mechanisms that are used by cells to increase or decrease the production of specific gene products. Sophisticated programs of gene expression are widely observed in biology, for example to trigger developmental pathways, respond to environmental stimuli, or adapt to new food sources. Virtually any step of gene expression can be modulated, from transcriptional initiation, to RNA processing, and to the post-translational modification of a protein. Often, one gene regulator controls another, and so on, in a gene regulatory network.

Mosaicism or genetic mosaicism is a condition in which a multicellular organism possesses more than one genetic line as the result of genetic mutation. This means that various genetic lines resulted from a single fertilized egg. Mosaicism is one of several possible causes of chimerism, wherein a single organism is composed of cells with more than one distinct genotype.

Spatiotemporal gene expression is the activation of genes within specific tissues of an organism at specific times during development. Gene activation patterns vary widely in complexity. Some are straightforward and static, such as the pattern of tubulin, which is expressed in all cells at all times in life. Some, on the other hand, are extraordinarily intricate and difficult to predict and model, with expression fluctuating wildly from minute to minute or from cell to cell. Spatiotemporal variation plays a key role in generating the diversity of cell types found in developed organisms; since the identity of a cell is specified by the collection of genes actively expressed within that cell, if gene expression was uniform spatially and temporally, there could be at most one kind of cell.

Two-hybrid screening is a molecular biology technique used to discover protein–protein interactions (PPIs) and protein–DNA interactions by testing for physical interactions between two proteins or a single protein and a DNA molecule, respectively.
Ectopic is a word used with a prefix, ecto, meaning “out of place.” Ectopic expression is an abnormal gene expression in a cell type, tissue type, or developmental stage in which the gene is not usually expressed. The term ectopic expression is predominantly used in studies using metazoans, especially in Drosophila melanogaster for research purposes.

In genetics, Flp-FRT recombination is a site-directed recombination technology, increasingly used to manipulate an organism's DNA under controlled conditions in vivo. It is analogous to Cre-lox recombination but involves the recombination of sequences between short flippase recognition target (FRT) sites by the recombinase flippase (Flp) derived from the 2 µ plasmid of baker's yeast Saccharomyces cerevisiae.
An enhancer trap is a method in molecular biology. The enhancer trap construct contains a transposable element and a reporter gene. The first is necessary for (random) insertion in the genome, the latter is necessary for identification of the spatial regulation by the enhancer. On top of this, the construct usually includes a genetic marker, e.g., the white gene producing red-colored eyes in Drosophila, or ampicillin resistance in E. coli.

Brainbow is a process by which individual neurons in the brain can be distinguished from neighboring neurons using fluorescent proteins. By randomly expressing different ratios of red, green, and blue derivatives of green fluorescent protein in individual neurons, it is possible to flag each neuron with a distinctive color. This process has been a major contribution to the field of neural connectomics.

Bioreporters are intact, living microbial cells that have been genetically engineered to produce a measurable signal in response to a specific chemical or physical agent in their environment. Bioreporters contain two essential genetic elements, a promoter gene and a reporter gene. The promoter gene is turned on (transcribed) when the target agent is present in the cell’s environment. The promoter gene in a normal bacterial cell is linked to other genes that are then likewise transcribed and then translated into proteins that help the cell in either combating or adapting to the agent to which it has been exposed. In the case of a bioreporter, these genes, or portions thereof, have been removed and replaced with a reporter gene. Consequently, turning on the promoter gene now causes the reporter gene to be turned on. Activation of the reporter gene leads to production of reporter proteins that ultimately generate some type of a detectable signal. Therefore, the presence of a signal indicates that the bioreporter has sensed a particular target agent in its environment.
Mosaic analysis with a repressible cell marker, or MARCM, is a genetics technique for creating individually labeled homozygous cells in an otherwise heterozygous Drosophila melanogaster. It has been a crucial tool in studying the development of the Drosophila nervous system. This technique relies on recombination during mitosis mediated by FLP-FRT recombination. As one copy of a gene, provided by the balancer chromosome, is often enough to rescue a mutant phenotype, MARCM clones can be used to study a mutant phenotype in an otherwise wildtype animal.
An upstream activating sequence or upstream activation sequence (UAS) is a cis-acting regulatory sequence. It is distinct from the promoter and increases the expression of a neighbouring gene. Due to its essential role in activating transcription, the upstream activating sequence is often considered to be analogous to the function of the enhancer in multicellular eukaryotes. Upstream activation sequences are a crucial part of induction, enhancing the expression of the protein of interest through increased transcriptional activity. The upstream activation sequence is found adjacently upstream to a minimal promoter and serves as a binding site for transactivators. If the transcriptional transactivator does not bind to the UAS in the proper orientation then transcription cannot begin. To further understand the function of an upstream activation sequence, it is beneficial to see its role in the cascade of events that lead to transcription activation. The pathway begins when activators bind to their target at the UAS recruiting a mediator. A TATA-binding protein subunit of a transcription factor then binds to the TATA box, recruiting additional transcription factors. The mediator then recruits RNA polymerase II to the pre-initiation complex. Once initiated, RNA polymerase II is released from the complex and transcription begins.
Andrea Hilary Brand is the Herchel Smith Professor of Molecular Biology and a Fellow of Jesus College, Cambridge. She heads a lab investigating nervous system development at the Gurdon Institute and the Department of Physiology, Development and Neuroscience. She developed the GAL4/UAS system with Norbert Perrimon which has been described as “a fly geneticist's Swiss army knife”.
Pigment dispersing factor (pdf) is a gene that encodes the protein PDF, which is part of a large family of neuropeptides. Its hormonal product, pigment dispersing hormone (PDH), was named for the diurnal pigment movement effect it has in crustacean retinal cells upon its initial discovery in the central nervous system of arthropods. The movement and aggregation of pigments in retina cells and extra-retinal cells is hypothesized to be under a split hormonal control mechanism. One hormonal set is responsible for concentrating chromatophoral pigment by responding to changes in the organism's exposure time to darkness. Another hormonal set is responsible for dispersion and responds to the light cycle. However, insect pdf genes do not function in such pigment migration since they lack the chromatophore.
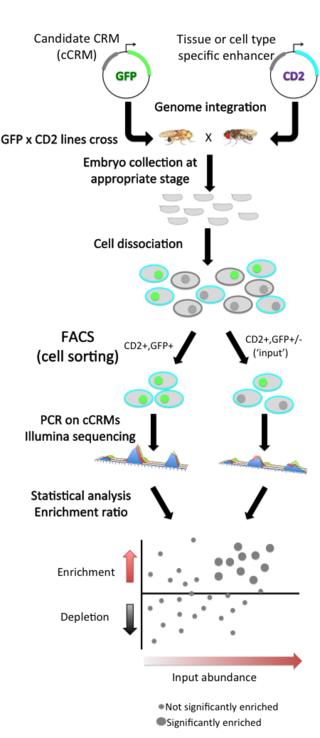
Enhancer-FACS-seq (eFS), developed by the Bulyk lab at Brigham and Women’s Hospital and Harvard Medical School, is a highly parallel enhancer assay that aims for the identification of active, tissue-specific transcriptional enhancers, in the context of whole Drosophila melanogaster embryos. This technology replaces the use of microscopy to screen for tissue-specific enhancers with fluorescence activated cell sorting (FACS) of dissociated cells from whole embryos, combined with identification by high-throughput Illumina sequencing.

Roger Brent is an American biologist known for his work on gene regulation and systems biology. He studies the quantitative behaviors of cell signaling systems and the origins and consequences of variation in them. He is Full Member in the Division of Basic Sciences at the Fred Hutchinson Cancer Research Center and an Affiliate Professor of Genome Sciences at the University of Washington.
Paul H. Taghert is an American chronobiologist known for pioneering research on the roles and regulation of neuropeptide signaling in the brain using Drosophila melanogaster as a model. He is a professor of neuroscience in the Department of Neuroscience at Washington University in St. Louis.
Q-system is a genetic tool that allows to express transgenes in a living organism. Originally the Q-system was developed for use in the vinegar fly Drosophila melanogaster, and was rapidly adapted for use in cultured mammalian cells, zebrafish, worms and mosquitoes. The Q-system utilizes genes from the qa cluster of the bread fungus Neurospora crassa, and consists of four components: the transcriptional activator (QF/QF2/QF2w), the enhancer QUAS, the repressor QS, and the chemical de-repressor quinic acid. Similarly to GAL4/UAS and LexA/LexAop, the Q-system is a binary expression system that allows to express reporters or effectors in a defined subpopulation of cells with the purpose of visualising these cells or altering their function. In addition, GAL4/UAS, LexA/LexAop and the Q-system function independently of each other and can be used simultaneously to achieve a desired pattern of reporter expression, or to express several reporters in different subsets of cells.
The Gal4 transcription factor is a positive regulator of gene expression of galactose-induced genes. This protein represents a large fungal family of transcription factors, Gal4 family, which includes over 50 members in the yeast Saccharomyces cerevisiae e.g. Oaf1, Pip2, Pdr1, Pdr3, Leu3.
Ravi Allada is an Indian-American chronobiologist studying the circadian and homeostatic regulation of sleep primarily in the fruit fly Drosophila. He is the Edward C. Stuntz Distinguished Professor of Neuroscience and Chair of the Department of Neurobiology at Northwestern University. Working with Michael Rosbash, he positionally cloned the Drosophila Clock gene. In his laboratory at Northwestern, he discovered a conserved mechanism for circadian control of sleep-wake cycle, as well as circuit mechanisms that manage levels of sleep.









