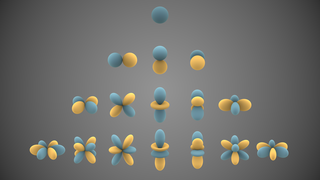In nuclear physics, atomic physics, and nuclear chemistry, the nuclear shell model is a model of the atomic nucleus which uses the Pauli exclusion principle to describe the structure of the nucleus in terms of energy levels. The first shell model was proposed by Dmitri Ivanenko in 1932. The model was developed in 1949 following independent work by several physicists, most notably Maria Goeppert Mayer and J. Hans D. Jensen, who shared half of the 1963 Nobel Prize in Physics for their contributions.

The quantum harmonic oscillator is the quantum-mechanical analog of the classical harmonic oscillator. Because an arbitrary smooth potential can usually be approximated as a harmonic potential at the vicinity of a stable equilibrium point, it is one of the most important model systems in quantum mechanics. Furthermore, it is one of the few quantum-mechanical systems for which an exact, analytical solution is known.

In physics, angular velocity, also known as angular frequency vector, is a pseudovector representation of how the angular position or orientation of an object changes with time, i.e. how quickly an object rotates around an axis of rotation and how fast the axis itself changes direction.
In physics, a phonon is a collective excitation in a periodic, elastic arrangement of atoms or molecules in condensed matter, specifically in solids and some liquids. A type of quasiparticle, a phonon is an excited state in the quantum mechanical quantization of the modes of vibrations for elastic structures of interacting particles. Phonons can be thought of as quantized sound waves, similar to photons as quantized light waves. However, photons are fundamental particles that can be individually detected, whereas phonons, being quasiparticles, are an emergent phenomenon.
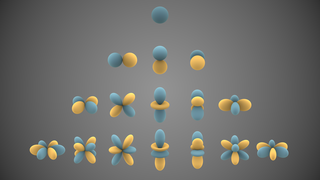
In mathematics and physical science, spherical harmonics are special functions defined on the surface of a sphere. They are often employed in solving partial differential equations in many scientific fields.
In physics, the Clebsch–Gordan (CG) coefficients are numbers that arise in angular momentum coupling in quantum mechanics. They appear as the expansion coefficients of total angular momentum eigenstates in an uncoupled tensor product basis. In more mathematical terms, the CG coefficients are used in representation theory, particularly of compact Lie groups, to perform the explicit direct sum decomposition of the tensor product of two irreducible representations. The name derives from the German mathematicians Alfred Clebsch and Paul Gordan, who encountered an equivalent problem in invariant theory.
The old quantum theory is a collection of results from the years 1900–1925 which predate modern quantum mechanics. The theory was never complete or self-consistent, but was rather a set of heuristic corrections to classical mechanics. The theory is now understood as the semi-classical approximation to modern quantum mechanics. The main and final accomplishments of the old quantum theory were the determination of the modern form of the periodic table by Edmund Stoner and the Pauli exclusion principle which were both premised on the Arnold Sommerfeld enhancements to the Bohr model of the atom.
In rotordynamics, the rigid rotor is a mechanical model of rotating systems. An arbitrary rigid rotor is a 3-dimensional rigid object, such as a top. To orient such an object in space requires three angles, known as Euler angles. A special rigid rotor is the linear rotor requiring only two angles to describe, for example of a diatomic molecule. More general molecules are 3-dimensional, such as water, ammonia, or methane.
In molecular physics, the molecular term symbol is a shorthand expression of the group representation and angular momenta that characterize the state of a molecule, i.e. its electronic quantum state which is an eigenstate of the electronic molecular Hamiltonian. It is the equivalent of the term symbol for the atomic case. However, the following presentation is restricted to the case of homonuclear diatomic molecules, or other symmetric molecules with an inversion centre. For heteronuclear diatomic molecules, the u/g symbol does not correspond to any exact symmetry of the electronic molecular Hamiltonian. In the case of less symmetric molecules the molecular term symbol contains the symbol of the group representation to which the molecular electronic state belongs.

The Duffing equation, named after Georg Duffing (1861–1944), is a non-linear second-order differential equation used to model certain damped and driven oscillators. The equation is given by
The Gross–Pitaevskii equation describes the ground state of a quantum system of identical bosons using the Hartree–Fock approximation and the pseudopotential interaction model.
In mathematics, vector spherical harmonics (VSH) are an extension of the scalar spherical harmonics for use with vector fields. The components of the VSH are complex-valued functions expressed in the spherical coordinate basis vectors.
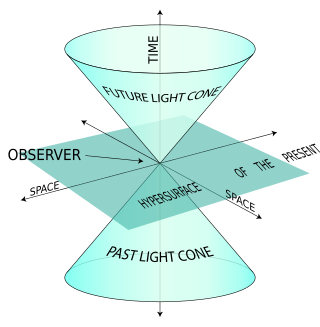
The light-front quantization of quantum field theories provides a useful alternative to ordinary equal-time quantization. In particular, it can lead to a relativistic description of bound systems in terms of quantum-mechanical wave functions. The quantization is based on the choice of light-front coordinates, where plays the role of time and the corresponding spatial coordinate is . Here, is the ordinary time, is one Cartesian coordinate, and is the speed of light. The other two Cartesian coordinates, and , are untouched and often called transverse or perpendicular, denoted by symbols of the type . The choice of the frame of reference where the time and -axis are defined can be left unspecified in an exactly soluble relativistic theory, but in practical calculations some choices may be more suitable than others.
In linear algebra, a raising or lowering operator is an operator that increases or decreases the eigenvalue of another operator. In quantum mechanics, the raising operator is sometimes called the creation operator, and the lowering operator the annihilation operator. Well-known applications of ladder operators in quantum mechanics are in the formalisms of the quantum harmonic oscillator and angular momentum.

Symmetries in quantum mechanics describe features of spacetime and particles which are unchanged under some transformation, in the context of quantum mechanics, relativistic quantum mechanics and quantum field theory, and with applications in the mathematical formulation of the standard model and condensed matter physics. In general, symmetry in physics, invariance, and conservation laws, are fundamentally important constraints for formulating physical theories and models. In practice, they are powerful methods for solving problems and predicting what can happen. While conservation laws do not always give the answer to the problem directly, they form the correct constraints and the first steps to solving a multitude of problems.
Ramsey interferometry, also known as the separated oscillating fields method, is a form of particle interferometry that uses the phenomenon of magnetic resonance to measure transition frequencies of particles. It was developed in 1949 by Norman Ramsey, who built upon the ideas of his mentor, Isidor Isaac Rabi, who initially developed a technique for measuring particle transition frequencies. Ramsey's method is used today in atomic clocks and in the S.I. definition of the second. Most precision atomic measurements, such as modern atom interferometers and quantum logic gates, have a Ramsey-type configuration. A more modern method, known as Ramsey–Bordé interferometry uses a Ramsey configuration and was developed by French physicist Christian Bordé and is known as the Ramsey–Bordé interferometer. Bordé's main idea was to use atomic recoil to create a beam splitter of different geometries for an atom-wave. The Ramsey–Bordé interferometer specifically uses two pairs of counter-propagating interaction waves, and another method named the "photon-echo" uses two co-propagating pairs of interaction waves.
Molecular symmetry in physics and chemistry describes the symmetry present in molecules and the classification of molecules according to their symmetry. Molecular symmetry is a fundamental concept in the application of Quantum Mechanics in physics and chemistry, for example it can be used to predict or explain many of a molecule's properties, such as its dipole moment and its allowed spectroscopic transitions, without doing the exact rigorous calculations. To do this it is necessary to classify the states of the molecule using the irreducible representations from the character table of the symmetry group of the molecule. Among all the molecular symmetries, diatomic molecules show some distinct features and they are relatively easier to analyze.

In nuclear physics and nuclear chemistry, the fission barrier is the activation energy required for a nucleus of an atom to undergo fission. This barrier may also be defined as the minimum amount of energy required to deform the nucleus to the point where it is irretrievably committed to the fission process. The energy to overcome this barrier can come from either neutron bombardment of the nucleus, where the additional energy from the neutron brings the nucleus to an excited state and undergoes deformation, or through spontaneous fission, where the nucleus is already in an excited and deformed state.
The Pomeranchuk instability is an instability in the shape of the Fermi surface of a material with interacting fermions, causing Landau’s Fermi liquid theory to break down. It occurs when a Landau parameter in Fermi liquid theory has a sufficiently negative value, causing deformations of the Fermi surface to be energetically favourable. It is named after the Soviet physicist Isaak Pomeranchuk.