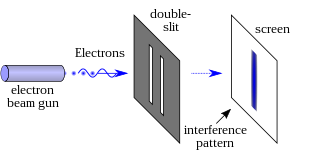
In modern physics, the double-slit experiment demonstrates that light and matter can satisfy the seemingly incongruous classical definitions for both waves and particles. This ambiguity is considered evidence for the fundamentally probabilistic nature of quantum mechanics. This type of experiment was first performed by Thomas Young in 1801, as a demonstration of the wave behavior of visible light. In 1927, Davisson and Germer and, independently George Paget Thomson and his research student Alexander Reid demonstrated that electrons show the same behavior, which was later extended to atoms and molecules. Thomas Young's experiment with light was part of classical physics long before the development of quantum mechanics and the concept of wave–particle duality. He believed it demonstrated that Christiaan Huygens' wave theory of light was correct, and his experiment is sometimes referred to as Young's experiment or Young's slits.

In quantum mechanics, Schrödinger's cat is a thought experiment, sometimes described as a paradox, of quantum superposition. In the thought experiment, a hypothetical cat may be considered simultaneously both alive and dead, while it is unobserved in a closed box, as a result of its fate being linked to a random subatomic event that may or may not occur. This thought experiment was devised by physicist Erwin Schrödinger in 1935 in a discussion with Albert Einstein to illustrate what Schrödinger saw as the problems of the Copenhagen interpretation of quantum mechanics.
Wave–particle duality is the concept in quantum mechanics that quantum entities exhibit particle or wave properties according to the experimental circumstances. It expresses the inability of the classical concepts such as particle or wave to fully describe the behavior of quantum objects. During the 19th and early 20th centuries, light was found to behave as a wave, and then later discovered to have a particulate character, whereas electrons were found to act as particles, and then later discovered to have wavelike aspects. The concept of duality arose to name these contradictions.
A timeline of atomic and subatomic physics.

An alpha particle X-ray spectrometer (APXS) is a spectrometer that analyses the chemical element composition of a sample from scattered alpha particles and fluorescent X-rays after a sample is irradiated with alpha particles and X-rays from radioactive sources. This method of analysing the elemental composition of a sample is most often used on space missions, which require low weight, small size, and minimal power consumption. Other methods are faster, and do not require the use of radioactive materials, but require larger equipment with greater power requirements. A variation is the alpha proton X-ray spectrometer, such as on the Pathfinder mission, which also detects protons.
In quantum mechanics, the measurement problem is the problem of how, or whether, wave function collapse occurs. The inability to observe such a collapse directly has given rise to different interpretations of quantum mechanics and poses a key set of questions that each interpretation must answer.
The Afshar experiment is a variation of the double-slit experiment in quantum mechanics, devised and carried out by Shahriar Afshar in 2004. In the experiment, light generated by a laser passes through two closely spaced pinholes, and is refocused by a lens so that the image of each pinhole falls on a separate single-photon detector. In addition, a grid of thin wires is placed just before the lens on the dark fringes of an interference pattern.
The Davisson–Germer experiment was a 1923-27 experiment by Clinton Davisson and Lester Germer at Western Electric, in which electrons, scattered by the surface of a crystal of nickel metal, displayed a diffraction pattern. This confirmed the hypothesis, advanced by Louis de Broglie in 1924, of wave-particle duality, and also the wave mechanics approach of the Schrödinger equation. It was an experimental milestone in the creation of quantum mechanics.
The Mott problem is an iconic challenge to quantum mechanics theory: how can the prediction of spherically symmetric wave function result in linear tracks seen in a cloud chamber. The problem was first formulated in 1927 by Albert Einstein and Max Born and solved in 1929 by Nevill Francis Mott. Mott's solution notably only uses the wave equation, not wavefunction collapse, and it is considered the earliest example of what is now called decoherence theory.
Quantum weirdness encompasses the aspects of quantum mechanics that challenge and defy human physical intuition based on the Newtonian mechanics of classical physics. These aspects include:
Quantum mechanics is the study of matter and its interactions with energy on the scale of atomic and subatomic particles. By contrast, classical physics explains matter and energy only on a scale familiar to human experience, including the behavior of astronomical bodies such as the moon. Classical physics is still used in much of modern science and technology. However, towards the end of the 19th century, scientists discovered phenomena in both the large (macro) and the small (micro) worlds that classical physics could not explain. The desire to resolve inconsistencies between observed phenomena and classical theory led to a revolution in physics, a shift in the original scientific paradigm: the development of quantum mechanics.
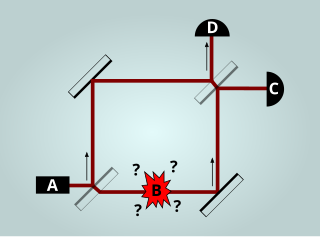
The Elitzur–Vaidman bomb-tester is a quantum mechanics thought experiment that uses interaction-free measurements to verify that a bomb is functional without having to detonate it. It was conceived in 1993 by Avshalom Elitzur and Lev Vaidman. Since their publication, real-world experiments have confirmed that their theoretical method works as predicted.
The ensemble interpretation of quantum mechanics considers the quantum state description to apply only to an ensemble of similarly prepared systems, rather than supposing that it exhaustively represents an individual physical system.
Objective-collapse theories, also known as models of spontaneous wave function collapse or dynamical reduction models, are proposed solutions to the measurement problem in quantum mechanics. As with other theories called interpretations of quantum mechanics, they are possible explanations of why and how quantum measurements always give definite outcomes, not a superposition of them as predicted by the Schrödinger equation, and more generally how the classical world emerges from quantum theory. The fundamental idea is that the unitary evolution of the wave function describing the state of a quantum system is approximate. It works well for microscopic systems, but progressively loses its validity when the mass / complexity of the system increases.
In physics, a quantum state space is an abstract space in which different "positions" represent, not literal locations, but rather quantum states of some physical system. It is the quantum analog of the phase space of classical mechanics.
In physics, the observer effect is the disturbance of an observed system by the act of observation. This is often the result of utilizing instruments that, by necessity, alter the state of what they measure in some manner. A common example is checking the pressure in an automobile tire, which causes some of the air to escape, thereby changing the pressure to observe it. Similarly, seeing non-luminous objects requires light hitting the object to cause it to reflect that light. While the effects of observation are often negligible, the object still experiences a change. This effect can be found in many domains of physics, but can usually be reduced to insignificance by using different instruments or observation techniques.

The atomic nucleus is the small, dense region consisting of protons and neutrons at the center of an atom, discovered in 1911 by Ernest Rutherford based on the 1909 Geiger–Marsden gold foil experiment. After the discovery of the neutron in 1932, models for a nucleus composed of protons and neutrons were quickly developed by Dmitri Ivanenko and Werner Heisenberg. An atom is composed of a positively charged nucleus, with a cloud of negatively charged electrons surrounding it, bound together by electrostatic force. Almost all of the mass of an atom is located in the nucleus, with a very small contribution from the electron cloud. Protons and neutrons are bound together to form a nucleus by the nuclear force.
Hardy's paradox is a thought experiment in quantum mechanics devised by Lucien Hardy in 1992–1993 in which a particle and its antiparticle may interact without annihilating each other.
The timeline of quantum mechanics is a list of key events in the history of quantum mechanics, quantum field theories and quantum chemistry.
Unbiquadium, also known as element 124 or eka-uranium, is a hypothetical chemical element; it has placeholder symbol Ubq and atomic number 124. Unbiquadium and Ubq are the temporary IUPAC name and symbol, respectively, until the element is discovered, confirmed, and a permanent name is decided upon. In the periodic table, unbiquadium is expected to be a g-block superactinide and the sixth element in the 8th period. Unbiquadium has attracted attention, as it may lie within the island of stability, leading to longer half-lives, especially for 308Ubq which is predicted to have a magic number of neutrons (184).





