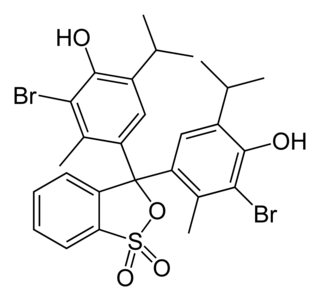
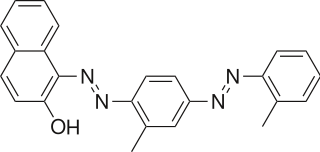
A solvent dye is a dye soluble in organic solvents. It is usually used as a solution in an organic solvent. [1]

A dye is a coloured substance that chemically bonds to the substrate to which it is being applied. This distinguishes dyes from pigments which do not chemically bind to the material they colour. The dye is generally applied in an aqueous solution, and may require a mordant to improve the fastness of the dye on the fiber.
Solvent dyes are used to color organic solvents, hydrocarbon fuels, waxes, lubricants, plastics, and other hydrocarbon-based nonpolar materials. Fuel dyes are one use of solvent dyes. Their molecules are typically nonpolar or little polar, and they do not undergo ionization. They are insoluble in water. They form a colloidal solution in solvents. They have poor (basic dyes) to good (metal complex based) light fastness.

Waxes are a diverse class of organic compounds that are lipophilic, malleable solids near ambient temperatures. They include higher alkanes and lipids, typically with melting points above about 40 °C (104 °F), melting to give low viscosity liquids. Waxes are insoluble in water but soluble in organic, nonpolar solvents. Natural waxes of different types are produced by plants and animals and occur in petroleum.
A lubricant is a substance, usually organic, introduced to reduce friction between surfaces in mutual contact, which ultimately reduces the heat generated when the surfaces move. It may also have the function of transmitting forces, transporting foreign particles, or heating or cooling the surfaces. The property of reducing friction is known as lubricity.

Plastic is material consisting of any of a wide range of synthetic or semi-synthetic organic compounds that are malleable and so can be molded into solid objects.
Solvent dyes are used for gold imitation (and other transparent metallic effects) of metallized polyester films. Also used in marking inks, inkjet inks, glass coloration, and so on.
Names of solvent dyes are often generic, of the scheme "solvent <color><number>", e.g. Solvent Red 24, Solvent Red 26, Solvent Red 164, Solvent Yellow 124, Solvent Blue 35, etc.
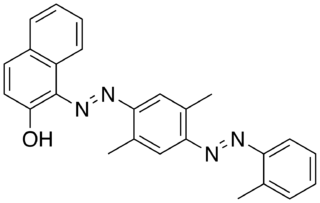
Solvent Red 26, also known as Oil Red EGN or C.I. 26120, is a purplish red synthetic azo dye. It is soluble in oils and insoluble in water.
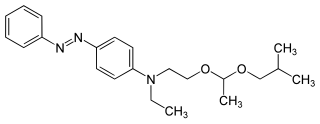
Solvent Yellow 124 is a yellow azo dye used in European Union as a fuel dye. It is a marker used since August 2002 to distinguish diesel fuel intended for heating from a higher-taxed motor diesel fuel. It is added to fuels not intended for motor vehicles in amounts of 6 mg/L or 7 mg/kg under the name Euromarker.
Red and yellow solvent dyes are often azo dyes, green and blue ones tend to be anthraquinone dyes.

Azo dyes are organic compounds bearing the functional group R−N=N−R′, in which R and R′ are usually aryl. They are a commercially important family of azo compounds, i.e. compounds containing the linkage C-N=N-C. Azo dyes are widely used to treat textiles, leather articles, and some foods. Chemically related to azo dyes are azo pigments, which are insoluble in water and other solvents.

Anthraquinone dyes are an abundant group of dyes comprising a anthraquinone unit as the shared structural element. Anthraquinone itself is colourless, but red to blue dyes are obtained by introducing electron donor groups such as hydroxy or amino groups in the 1-, 4-, 5- or 8-position. Anthraquinone dyestuffs are structurally related to indigo dyestuffs and are classified together with these in the group of carbonyl dyes.