In cellular biology, paracrine signaling is a form of cell signaling, a type of cellular communication in which a cell produces a signal to induce changes in nearby cells, altering the behaviour of those cells. Signaling molecules known as paracrine factors diffuse over a relatively short distance, as opposed to cell signaling by endocrine factors, hormones which travel considerably longer distances via the circulatory system; juxtacrine interactions; and autocrine signaling. Cells that produce paracrine factors secrete them into the immediate extracellular environment. Factors then travel to nearby cells in which the gradient of factor received determines the outcome. However, the exact distance that paracrine factors can travel is not certain.
The Wnt signaling pathways are a group of signal transduction pathways which begin with proteins that pass signals into a cell through cell surface receptors. The name Wnt is a portmanteau created from the names Wingless and Int-1. Wnt signaling pathways use either nearby cell-cell communication (paracrine) or same-cell communication (autocrine). They are highly evolutionarily conserved in animals, which means they are similar across animal species from fruit flies to humans.
Frzb is a Wnt-binding protein especially important in embryonic development. It is a competitor for the cell-surface G-protein receptor Frizzled.
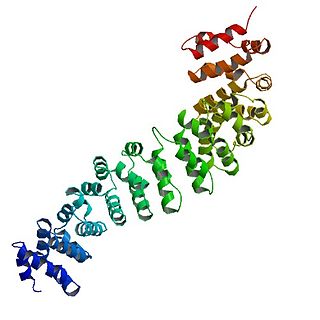
Catenin beta-1, also known as β-catenin (beta-catenin), is a protein that in humans is encoded by the CTNNB1 gene.
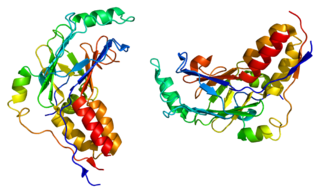
Mothers against decapentaplegic homolog 3 also known as SMAD family member 3 or SMAD3 is a protein that in humans is encoded by the SMAD3 gene.

α-Catenin (alpha-catenin) functions as the primary protein link between cadherins and the actin cytoskeleton. It has been reported that the actin binding proteins vinculin and α-actinin can bind to alpha-catenin. It has been suggested that alpha-catenin does not bind with high affinity to both actin filaments and the E-cadherin-beta-catenin complex at the same time. It has been observed that when α-catenin is not in a molecular complex with β-catenin, it dimerizes and functions to regulate actin filament assembly, possibly by competing with Arp2/3 protein. α-Catenin exhibits significant protein dynamics. However, a protein complex including a cadherin, actin, β-catenin and α-catenin has not been isolated.

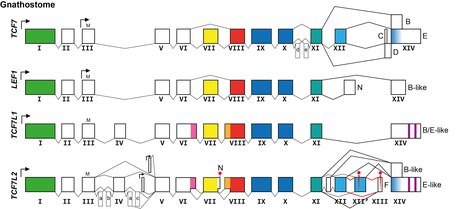
Transcription factor 7-like 2 , also known as TCF7L2 or TCF4, is a protein acting as a transcription factor that, in humans, is encoded by the TCF7L2 gene. The TCF7L2 gene is located on chromosome 10q25.2–q25.3, contains 19 exons. As a member of the TCF family, TCF7L2 can form a bipartite transcription factor and influence several biological pathways, including the Wnt signalling pathway.

Axin-1 is a protein that in humans is encoded by the AXIN1 gene.

Lymphoid enhancer-binding factor 1 (LEF1) is a protein that in humans is encoded by the LEF1 gene. It is a member of T cell factor/lymphoid enhancer factor (TCF/LEF) family.

Segment polarity protein dishevelled homolog DVL-1 is a protein that in humans is encoded by the DVL1 gene.

Protein Wnt-3a is a protein that in humans is encoded by the WNT3A gene.

Transcriptional regulator Kaiso is a protein that in humans is encoded by the ZBTB33 gene. This gene encodes a transcriptional regulator with bimodal DNA-binding specificity, which binds to methylated CGCG and also to the non-methylated consensus KAISO-binding site TCCTGCNA. The protein contains an N-terminal POZ/BTB domain and 3 C-terminal zinc finger motifs. It recruits the N-CoR repressor complex to promote histone deacetylation and the formation of repressive chromatin structures in target gene promoters. It may contribute to the repression of target genes of the Wnt signaling pathway, and may also activate transcription of a subset of target genes by the recruitment of catenin delta-2 (CTNND2). Its interaction with catenin delta-1 (CTNND1) inhibits binding to both methylated and non-methylated DNA. It also interacts directly with the nuclear import receptor Importin-α2, which may mediate nuclear import of this protein. Alternatively spliced transcript variants encoding the same protein have been identified.

Wnt inhibitory factor 1 is a protein that in humans is encoded by the WIF1 gene. WIF1 is a lipid-binding protein that binds to Wnt proteins and prevents them from triggering signalling.

Transcription factor 7 is the gene that in humans encodes for the TCF1 protein.

Chromodomain-helicase-DNA-binding protein 8 is an enzyme that in humans is encoded by the CHD8 gene.

B-cell CLL/lymphoma 9 protein is a protein that in humans is encoded by the BCL9 gene.

Dishevelled (Dsh) is a family of proteins involved in canonical and non-canonical Wnt signalling pathways. Dsh is a cytoplasmic phosphoprotein that acts directly downstream of frizzled receptors. It takes its name from its initial discovery in flies, where a mutation in the dishevelled gene was observed to cause improper orientation of body and wing hairs. There are vertebrate homologs in zebrafish, Xenopus (Xdsh), mice and humans. Dsh relays complex Wnt signals in tissues and cells, in normal and abnormal contexts. It is thought to interact with the SPATS1 protein when regulating the Wnt Signalling pathway.
Reptin is a tumor repressor protein that is a member of the ATPases Associated with various cellular Activities (AAA+) helicase family and regulates KAI1. Desumoylation of reptin alters the repressive function of reptin and its association with HDAC1. The sumoylation status of reptin modulates the invasive activity of cancer cells with metastatic potential. Reptin was reported in 2010 to be a good marker for metastasis. Another name for reptin, RuvB-like 2 comes from its similarity to RuvB, an ATP-dependent helicase found in bacteria. Reptin is highly conserved, being found in yeast, drosophila, and humans. It presents itself as a member of a number of different protein complexes, most of which function in chromatin modification, including PRC1, TIP60/NuA4 and INO80. Hence, it also has the names INO80J, TIP48, and TIP49B. In the majority of its functions, reptin is paired with a very similar protein, pontin (RUVBL1).

Transcription factor 7-like 1, also known as TCF7L1, is a human gene.

Dishevelled binding antagonist of beta catenin 1 is a protein that in humans is encoded by the DACT1 gene. Dact1 was originally described in 2002 as a negative regulator of Wnt signaling by binding and destabilizing Dishevelled. More recent investigation into the molecular function of Dact1 has identified its principle role in the cell as a scaffold to generate membrane-less biomolecular condensates through liquid-liquid phase separation. Mutations in the phase-separating regions of Dact1 lead to Townes-Brock Syndrome 2 while its overexpression is associated with bone metastasis.