Related Research Articles

Inorganic chemistry deals with synthesis and behavior of inorganic and organometallic compounds. This field covers chemical compounds that are not carbon-based, which are the subjects of organic chemistry. The distinction between the two disciplines is far from absolute, as there is much overlap in the subdiscipline of organometallic chemistry. It has applications in every aspect of the chemical industry, including catalysis, materials science, pigments, surfactants, coatings, medications, fuels, and agriculture.

Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and sometimes broadened to include metalloids like boron, silicon, and selenium, as well. Aside from bonds to organyl fragments or molecules, bonds to 'inorganic' carbon, like carbon monoxide, cyanide, or carbide, are generally considered to be organometallic as well. Some related compounds such as transition metal hydrides and metal phosphine complexes are often included in discussions of organometallic compounds, though strictly speaking, they are not necessarily organometallic. The related but distinct term "metalorganic compound" refers to metal-containing compounds lacking direct metal-carbon bonds but which contain organic ligands. Metal β-diketonates, alkoxides, dialkylamides, and metal phosphine complexes are representative members of this class. The field of organometallic chemistry combines aspects of traditional inorganic and organic chemistry.
Ferrocene is an organometallic compound with the formula Fe(C5H5)2. The molecule is a complex consisting of two cyclopentadienyl rings bound to a central iron atom. It is an orange solid with a camphor-like odor, that sublimes above room temperature, and is soluble in most organic solvents. It is remarkable for its stability: it is unaffected by air, water, strong bases, and can be heated to 400 °C without decomposition. In oxidizing conditions it can reversibly react with strong acids to form the ferrocenium cation Fe(C5H5)+2.
Cyclopentadiene is an organic compound with the formula C5H6. It is often abbreviated CpH because the cyclopentadienyl anion is abbreviated Cp−.

In organometallic chemistry, organolithium reagents are chemical compounds that contain carbon–lithium (C–Li) bonds. These reagents are important in organic synthesis, and are frequently used to transfer the organic group or the lithium atom to the substrates in synthetic steps, through nucleophilic addition or simple deprotonation. Organolithium reagents are used in industry as an initiator for anionic polymerization, which leads to the production of various elastomers. They have also been applied in asymmetric synthesis in the pharmaceutical industry. Due to the large difference in electronegativity between the carbon atom and the lithium atom, the C−Li bond is highly ionic. Owing to the polar nature of the C−Li bond, organolithium reagents are good nucleophiles and strong bases. For laboratory organic synthesis, many organolithium reagents are commercially available in solution form. These reagents are highly reactive, and are sometimes pyrophoric.

A triple bond in chemistry is a chemical bond between two atoms involving six bonding electrons instead of the usual two in a covalent single bond. Triple bonds are stronger than the equivalent single bonds or double bonds, with a bond order of three. The most common triple bond is in a nitrogen N2 molecule; the second most common is that between two carbon atoms, which can be found in alkynes. Other functional groups containing a triple bond are cyanides and isocyanides. Some diatomic molecules, such as dinitrogen and carbon monoxide, are also triple bonded. In skeletal formulae the triple bond is drawn as three parallel lines (≡) between the two connected atoms.

A silabenzene is a heteroaromatic compound containing one or more silicon atoms instead of carbon atoms in benzene. A single substitution gives silabenzene proper; additional substitutions give a disilabenzene, trisilabenzene, etc.

Organoboron chemistry or organoborane chemistry is the chemistry of organoboron compounds or organoboranes, which are chemical compounds of boron and carbon that are organic derivatives of borane (BH3), for example trialkyl boranes..

Michael James Steuart Dewar was an American theoretical chemist.

Frank Henry Westheimer NAS ForMemRS APS was an American chemist. He taught at the University of Chicago from 1936 to 1954, and at Harvard University from 1953 to 1983, becoming the Morris Loeb Professor of Chemistry in 1960, and Professor Emeritus in 1983. The Westheimer medal was established in his honor in 2002.

Henry Stephen Rzepa is a chemist and Emeritus Professor of Computational chemistry at Imperial College London.
Uranocene, U(C8H8)2, is an organouranium compound composed of a uranium atom sandwiched between two cyclooctatetraenide rings. It was one of the first organoactinide compounds to be synthesized. It is a green air-sensitive solid that dissolves in organic solvents. Uranocene, a member of the "actinocenes," a group of metallocenes incorporating elements from the actinide series. It is the most studied bis[8]annulene-metal system, although it has no known practical applications.
Eamonn Francis Healy is an Irish-American professor of chemistry, organic chemistry, and biochemistry at St. Edward's University in Austin, Texas, where his research focuses on the design of structure-activity probes to elucidate enzymatic activity. Targets include HIV-1 integrase, the c-Kit and src-abl proteins, and the metalloproteinases associated with CXCL16 shedding.

Dimanganese decacarbonyl, which has the chemical formula Mn2(CO)10, is a binary bimetallic carbonyl complex centered around the first row transition metal manganese. The first reported synthesis of Mn2(CO)10 was in 1954 at Linde Air Products Company and was performed by Brimm, Lynch, and Sesny. Their hypothesis about, and synthesis of, dimanganese decacarbonyl was fundamentally guided by the previously known dirhenium decacarbonyl (Re2(CO)10), the heavy atom analogue of Mn2(CO)10. Since its first synthesis, Mn2(CO)10 has been use sparingly as a reagent in the synthesis of other chemical species, but has found the most use as a simple system on which to study fundamental chemical and physical phenomena, most notably, the metal-metal bond. Dimanganese decacarbonyl is also used as a classic example to reinforce fundamental topics in organometallic chemistry like d-electron count, the 18-electron rule, oxidation state, valency, and the isolobal analogy.

Group 2 organometallic chemistry refers to the chemistry of compounds containing carbon bonded to any group 2 element. By far the most common group 2 organometallic compounds are the magnesium-containing Grignard reagents which are widely used in organic chemistry. Other organmetallic group 2 compounds are rare and are typically limited to academic interests.
Physical organic chemistry, a term coined by Louis Hammett in 1940, refers to a discipline of organic chemistry that focuses on the relationship between chemical structures and reactivity, in particular, applying experimental tools of physical chemistry to the study of organic molecules. Specific focal points of study include the rates of organic reactions, the relative chemical stabilities of the starting materials, reactive intermediates, transition states, and products of chemical reactions, and non-covalent aspects of solvation and molecular interactions that influence chemical reactivity. Such studies provide theoretical and practical frameworks to understand how changes in structure in solution or solid-state contexts impact reaction mechanism and rate for each organic reaction of interest.
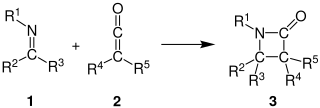
The Staudinger synthesis, also called the Staudinger ketene-imine cycloaddition, is a chemical synthesis in which an imine 1 reacts with a ketene 2 through a non-photochemical 2+2 cycloaddition to produce a β-lactam3. The reaction carries particular importance in the synthesis of β-lactam antibiotics. The Staudinger synthesis should not be confused with the Staudinger reaction, a phosphine or phosphite reaction used to reduce azides to amines.

Iron tetracarbonyl dihydride is the organometallic compound with the formula H2Fe(CO)4. This compound was the first transition metal hydride discovered. The complex is stable at low temperatures but decomposes rapidly at temperatures above –20 °C.

Clémence Corminboeuf is a Swiss chemist who is Professor of Computational chemistry at the École Polytechnique Fédérale de Lausanne. She was awarded the Swiss Chemical Society 2021 Heilbronner-Hückel Award.

Nitrogen pentahydride, also known as ammonium hydride is a hypothetical compound with the chemical formula NH5. There are two theoretical structures of nitrogen pentahydride. One structure is trigonal bipyramidal molecular geometry type NH5 molecule. Its nitrogen atom and hydrogen atoms are covalently bounded, and its symmetry group is D3h. Another predicted structure of nitrogen pentahydride is an ionic compound, make up of an ammonium ion and a hydride ion (NH4+H−). Until now, no one has synthesized this substance, or proved its existence, and related experiments have not directly observed nitrogen pentahydride. It is only speculated that it may be a reactive intermediate based on reaction products. Theoretical calculations show this molecule is thermodynamically unstable. The reason might be similar to the instability of nitrogen pentafluoride, so the possibility of its existence is low. However, nitrogen pentahydride might exist in special conditions or high pressure. Nitrogen pentahydride was considered for use as a solid rocket fuel for research in 1966.