Related Research Articles

Beryllium is a chemical element with the symbol Be and atomic number 4. It is a steel-gray, strong, lightweight and brittle alkaline earth metal. It is a divalent element that occurs naturally only in combination with other elements to form minerals. Notable gemstones high in beryllium include beryl and chrysoberyl. It is a relatively rare element in the universe, usually occurring as a product of the spallation of larger atomic nuclei that have collided with cosmic rays. Within the cores of stars, beryllium is depleted as it is fused into heavier elements. Beryllium constitutes about 0.0004 percent by mass of Earth's crust. The world's annual beryllium production of 220 tons is usually manufactured by extraction from the mineral beryl, a difficult process because beryllium bonds strongly to oxygen.

In chemistry, a heteroatom is, strictly, any atom that is not carbon or hydrogen.

The alkaline earth metals are six chemical elements in group 2 of the periodic table. They are beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra). The elements have very similar properties: they are all shiny, silvery-white, somewhat reactive metals at standard temperature and pressure.
In chemistry, an amphoteric compound is a molecule or ion that can react both as an acid and as a base. What exactly this can mean depends on which definitions of acids and bases are being used.

Berylliosis, or chronic beryllium disease (CBD), is a chronic allergic-type lung response and chronic lung disease caused by exposure to beryllium and its compounds, a form of beryllium poisoning. It is distinct from acute beryllium poisoning, which became rare following occupational exposure limits established around 1950. Berylliosis is an occupational lung disease.
A period 2 element is one of the chemical elements in the second row of the periodic table of the chemical elements. The periodic table is laid out in rows to illustrate recurring (periodic) trends in the chemical behavior of the elements as their atomic number increases; a new row is started when chemical behavior begins to repeat, creating columns of elements with similar properties.

Beryllium fluoride is the inorganic compound with the formula BeF2. This white solid is the principal precursor for the manufacture of beryllium metal. Its structure resembles that of quartz, but BeF2 is highly soluble in water.

Beryllium oxide (BeO), also known as beryllia, is an inorganic compound with the formula BeO. This colourless solid is a notable electrical insulator with a higher thermal conductivity than any other non-metal except diamond, and exceeds that of most metals. As an amorphous solid, beryllium oxide is white. Its high melting point leads to its use as a refractory material. It occurs in nature as the mineral bromellite. Historically and in materials science, beryllium oxide was called glucina or glucinium oxide, owing to its sweet taste.

Beryllium hydroxide, Be(OH)2, is an amphoteric hydroxide, dissolving in both acids and alkalis. Industrially, it is produced as a by-product in the extraction of beryllium metal from the ores beryl and bertrandite. The natural pure beryllium hydroxide is rare (in form of the mineral behoite, orthorhombic) or very rare (clinobehoite, monoclinic). When alkali is added to beryllium salt solutions the α-form (a gel) is formed. If this left to stand or boiled, the rhombic β-form precipitates. This has the same structure as zinc hydroxide, Zn(OH)2, with tetrahedral beryllium centers.

Basic beryllium acetate is the chemical compound with the formula Be4O(O2CCH3)6. This compound adopts a distinctive structure, but it has no applications and has been only lightly studied. It is a colourless solid that is soluble in organic solvents.

Beryllium chloride is an inorganic compound with the formula BeCl2. It is a colourless, hygroscopic solid that dissolves well in many polar solvents. Its properties are similar to those of aluminium chloride, due to beryllium's diagonal relationship with aluminium.
The Experimental Beryllium Oxide Reactor (EBOR) was a 10MWt helium cooled beryllium moderated nuclear reactor at Idaho National Laboratory. It never achieved criticality. The project started on February 17, 1958 as the Maritime Gas-Cooled Reactor. The project started with a contract between the U.S. Atomic Energy Commission and General Dynamics. The Goal of the project was to create a small nuclear reactor for use in merchant shipping or in a medium sized power plant. The main goals for the reactor were a simple design, low maintenance costs and maximum efficiency over a wide range of power settings. In December 1960 the project was authorized to construct a 10-Mw test reactor to determine the characteristics of the Beryllium Oxide gas cooled system. The EBOR was designed to test the basic fuel element and moderator designs for the final reactor. The EBOR used a Helium cooling system and was an intermediate step towards a prototype power plant. The plan was to use a closed cycle turbine or a steam cycle with the reactor to make a small land based or maritime power plant. This plan was abandoned as the reactor never achieved criticality.

Beryllium bromide is the chemical compound with the formula BeBr2. It is very hygroscopic and dissolves well in water. The compound is a polymer with tetrahedral coordinated Be centres.
Beryllium carbide, or Be2C, is a metal carbide. Similar to diamond, it is a very hard compound. It is used in nuclear reactors as a core material.
E-Material, also called E Material, is a metal matrix composite consisting of beryllium matrix with beryllium oxide particles. It has high thermal conductivity, and its thermal expansion can be adjusted to match other materials, e.g. silicon and gallium arsenide chips and various ceramics. It is chiefly used in microelectronics as substrate for power semiconductor devices and high density multi-chip modules, where it aids with removal of waste heat. E-materials have low weight and high strength, making them especially suitable for aerospace technology. Their high elastic modulus is favorable for absorbing vibrations and lowering material fatigue of attached modules and wire bonds.
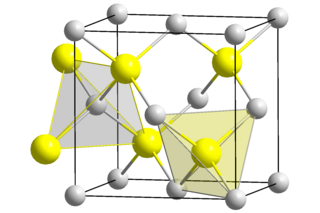
Beryllium sulfide (BeS) is an ionic compound from the sulfide group with the formula BeS. It is a white solid with a sphalerite structure that is decomposed by water and acids.
Beryllium poisoning is poisoning by the toxic effects of beryllium, or more usually its compounds. It takes two forms:

Beryllium oxalate is an inorganic compound, a salt of beryllium metal and oxalic acid with the chemical formula C
2BeO
4. It forms colorless crystals, dissolves in water, and also forms crystalline hydrates. The compound is used to prepare ultra-pure beryllium oxide by thermal decomposition.

Organoberyllium chemistry involves the synthesis and properties of organometallic compounds featuring the group 2 alkaline earth metal beryllium (Be). Beryllium is best known to have a +2 oxidation state and one of the smallest atoms and it is understudied in the periodic table. While metallic beryllium is relatively unreactive, its dust causes berylliosis and compounds are toxic. The Be2+ cation is characterized by the highest known charge density (Z/r = 6.45), making it one of the hardest cations and a very strong Lewis acid. It is most commonly used to coordinate other elements and can portray many types of compound through different ligands attachment. Coordination in beryllium can range from a coordination number of two to four. Most common ligands attached to beryllium are halides, hydride (like beryllium borohydride in a three-center two-electron bond), methyl, aryl, and alkyl. Beryllium can form complexes with known organic compounds such as phosphines, N-hetereocyclic carbenes (NHC), cyclic alkyl amino carbenes (CAAC), and β-diketiminates (NacNac). They can best be prepared by transmetallation or alkylation of beryllium chloride.
References
- Except where noted otherwise, data relate to Standard temperature and pressure.
- Reliability of data general note.