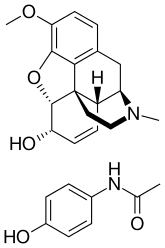
Hydrocodone, also known as dihydrocodeinone, is a semisynthetic opioid used to treat pain and as a cough suppressant. It is taken by mouth. Typically it is dispensed as the combination acetaminophen/hydrocodone or ibuprofen/hydrocodone for pain severe enough to require an opioid and in combination with homatropine methylbromide to relieve cough. It is also available by itself in a long-acting form under the brand name Zohydro ER, among others, to treat severe pain of a prolonged duration. Hydrocodone is a controlled drug: in the United States a Schedule II Controlled Substance.
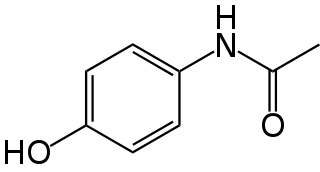
Paracetamol (acetaminophen) is a non-opioid analgesic and antipyretic agent used to treat fever and mild to moderate pain. It is a widely used over the counter medication. Common brand names include Tylenol and Panadol.

Tylenol is a brand of medication, advertised for reducing pain, reducing fever, and relieving the symptoms of allergies, cold, cough, headache, and influenza. The active ingredient of its original flagship product is paracetamol, an analgesic and antipyretic. Like the words paracetamol and acetaminophen, the brand name Tylenol is derived from a chemical name for the compound, N-acetyl-para-aminophenol (APAP). The brand name is owned by McNeil Consumer Healthcare, a subsidiary of Kenvue.
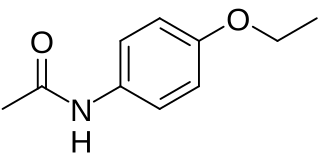
Phenacetin is a pain-relieving and fever-reducing drug, which was widely used following its introduction in 1887. It was withdrawn from medicinal use as dangerous from the 1970s.
Oxycodone/aspirin is a combination drug marketed by Endo Pharmaceuticals. It is a tablet containing a mixture of 325 mg of aspirin and 4.8355 mg of oxycodone HCl ; it is an opioid/non-opioid combination used to treat moderate to moderately severe pain. The safety of the combination during pregnancy has not been established, although aspirin is generally contraindicated during pregnancy, and the drug has been placed in pregnancy category D. Inactive ingredients include D&C Yellow 10, FD&C Yellow 6, microcrystalline cellulose, and corn starch. Percodan was first marketed by DuPont Pharmaceuticals and prescribed in the United States in 1950. Once a widely prescribed painkiller, it has largely been replaced by alternative oxycodone compounds containing paracetamol (acetaminophen) instead of aspirin, such as Percocet.
Oxycodone/paracetamol, sold under the brand name Percocet among others, is a fixed-dose combination of the opioid oxycodone with paracetamol (acetaminophen), used to treat moderate to severe pain.

Guaifenesin, also known as glyceryl guaiacolate, is an expectorant medication taken by mouth and marketed as an aid to eliminate sputum from the respiratory tract. Chemically, it is an ether of guaiacol and glycerine. It may be used in combination with other medications. A 2014 study found that guaifenesin has no effect on sputum production or clearance in upper respiratory infections.

Dihydrocodeine is a semi-synthetic opioid analgesic prescribed for pain or severe dyspnea, or as an antitussive, either alone or compounded with paracetamol (acetaminophen) or aspirin. It was developed in Germany in 1908 and first marketed in 1911.

Dextropropoxyphene is an analgesic in the opioid category, patented in 1955 and manufactured by Eli Lilly and Company. It is an optical isomer of levopropoxyphene. It is intended to treat mild pain and also has antitussive and local anaesthetic effects. The drug has been taken off the market in Europe and the US due to concerns of fatal overdoses and heart arrhythmias. It is still available in Australia, albeit with restrictions after an application by its manufacturer to review its proposed banning. Its onset of analgesia is said to be 20–30 minutes and peak effects are seen about 1.5–2.0 hours after oral administration.
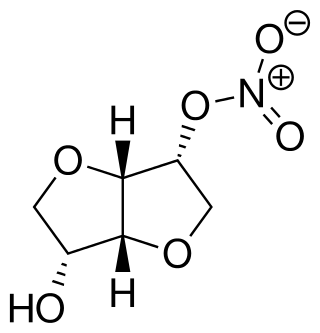
Isosorbide mononitrate, sold under many brand names, is a medication used for heart-related chest pain (angina), heart failure and esophageal spasms. It can be used both to treat and to prevent heart-related chest pain; however, it is generally less preferred than beta blockers or calcium channel blockers. It is taken by mouth.
Butalbital/acetaminophen, sold under the brand name Butapap among others, is a combination medication used to treat tension headaches and migraine headaches. It contains butalbital, a barbiturate and paracetamol (acetaminophen), an analgesic. Versions also containing caffeine are sold under the brand name Fioricet among others. It is taken by mouth. The combination is also sold with codeine.

Butalbital is a barbiturate with an intermediate duration of action. Butalbital is often combined with other medications, such as paracetamol (acetaminophen) or aspirin, for the treatment of pain and headache. The various formulations combined with codeine are FDA-approved for the treatment of tension headaches. Butalbital has the same chemical formula as talbutal but a different structure—one that presents as 5-allyl-5-isobutylbarbituric acid.

Hydrocodone/paracetamol is the combination of the pain medications hydrocodone and paracetamol (acetaminophen). It is used to treat moderate to severe pain. It is taken by mouth. Recreational use is common in the United States.

Codeine is an opiate and prodrug of morphine mainly used to treat pain, coughing, and diarrhea. It is also commonly used as a recreational drug. It is found naturally in the sap of the opium poppy, Papaver somniferum. It is typically used to treat mild to moderate degrees of pain. Greater benefit may occur when combined with paracetamol (acetaminophen) or a nonsteroidal anti-inflammatory drug (NSAID) such as aspirin or ibuprofen. Evidence does not support its use for acute cough suppression in children or adults. In Europe, it is not recommended as a cough medicine in those under 12 years of age. It is generally taken by mouth. It typically starts working after half an hour, with maximum effect at two hours. Its effects last for about four to six hours. Codeine exhibits abuse potential similar to other opioid medications, including a risk of habituation and overdose.
Insulin detemir, sold under the brand name Levemir among others, is a long-acting modified form of medical insulin used to treat both type 1 and type 2 diabetes. It is used by injection under the skin. It is effective for up to 24 hours.

Linagliptin, sold under the brand name Tradjenta among others, is a medication used to treat type 2 diabetes in conjunction with exercise and diet. It is generally less preferred than metformin and sulfonylureas as an initial treatment. It is taken by mouth.

Paracetamol poisoning, also known as acetaminophen poisoning, is caused by excessive use of the medication paracetamol (acetaminophen). Most people have few or non-specific symptoms in the first 24 hours following overdose. These symptoms include feeling tired, abdominal pain, or nausea. This is typically followed by absence of symptoms for a couple of days, after which yellowish skin, blood clotting problems, and confusion occurs as a result of liver failure. Additional complications may include kidney failure, pancreatitis, low blood sugar, and lactic acidosis. If death does not occur, people tend to recover fully over a couple of weeks. Without treatment, death from toxicity occurs 4 to 18 days later.
Ibuprofen/paracetamol, sold under the brand name Combogesic among others, is a fixed-dose combination of two medications, ibuprofen, a non-steroidal anti-inflammatory drug (NSAID); and paracetamol (acetaminophen), an analgesic and antipyretic. It is available as a generic medication.
Tramadol/paracetamol, also known as tramadol/acetaminophen and sold under the brand name Ultracet among others, is a fixed-dose combination medication used for the treatment of moderate to severe pain. It contains tramadol, as the hydrochloride, an analgesic; and paracetamol an analgesic. It is taken by mouth.
Guaifenesin/codeine is a fixed-dose combination cold medicine used for the treatment of cough. It contains guaifenesin, an expectorant; and codeine, as the phosphate, an opioid antitussive. It is taken by mouth.