
Creatinine is a breakdown product of creatine phosphate in muscle, and is usually produced at a fairly constant rate by the body.

Phosphocreatine, also known as creatine phosphate (CP) or PCr (Pcr), is a phosphorylated creatine molecule that serves as a rapidly mobilizable reserve of high-energy phosphates in skeletal muscle and the brain to recycle adenosine triphosphate, the energy currency of the cell.
Methyl formate, also called methyl methanoate, is the methyl ester of formic acid. The simplest example of an ester, it is a colorless liquid with an ethereal odour, high vapor pressure, and low surface tension. It is a precursor to many other compounds of commercial interest.

Metenolone, or methenolone, is an androgen and anabolic steroid (AAS) which is used in the form of esters such as metenolone acetate and metenolone enanthate. Metenolone esters are used mainly in the treatment of anemia due to bone marrow failure. Metenolone acetate is taken by mouth, while metenolone enanthate is given by injection into muscle.

N-Methylethanolamine is an alkanolamine with the formula CH3NHCH2CH2OH. It is flammable, corrosive, colorless, viscous liquid. It is an intermediate in the biosynthesis of choline.

Methyl acetate, also known as MeOAc, acetic acid methyl ester or methyl ethanoate, is a carboxylate ester with the formula CH3COOCH3. It is a flammable liquid with a characteristically pleasant smell reminiscent of some glues and nail polish removers. Methyl acetate is occasionally used as a solvent, being weakly polar and lipophilic, but its close relative ethyl acetate is a more common solvent being less toxic and less soluble in water. Methyl acetate has a solubility of 25% in water at room temperature. At elevated temperature its solubility in water is much higher. Methyl acetate is not stable in the presence of strong aqueous bases or aqueous acids. Methyl acetate is not considered as a VOC in the USA.

Methyl butyrate, also known under the systematic name methyl butanoate, is the methyl ester of butyric acid. Like most esters, it has a fruity odor, in this case resembling apples or pineapples. At room temperature, it is a colorless liquid with low solubility in water, upon which it floats to form an oily layer. Although it is flammable, it has a relatively low vapor pressure, so it can be safely handled at room temperature without special safety precautions.
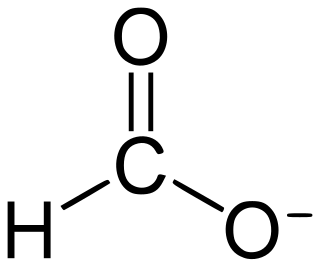
Formate (IUPAC name: methanoate) is the anion derived from formic acid. Its formula is represented in various equivalent ways: HCOO− or CHOO− or HCO2−. It is the product of deprotonation of formic acid. It is the simplest carboxylate anion. A formate (compound) is a salt or ester of formic acid.
Heptanoic acid, also called enanthic acid, is an organic compound composed of a seven-carbon chain terminating in a carboxylic acid. It is an oily liquid with an unpleasant, rancid odor. It contributes to the odor of some rancid oils. It is slightly soluble in water, but very soluble in ethanol and ether.

Trestolone acetate is a synthetic and injected anabolic–androgenic steroid (AAS) and a derivative of nandrolone (19-nortestosterone) which was never marketed. It is an androgen ester – specifically, the C17 acetate ester of trestolone.

Glycocyamine is a metabolite of glycine in which the amino group has been converted into a guanidine by guanylation. In vertebrate organism it is then transformed into creatine by methylation.
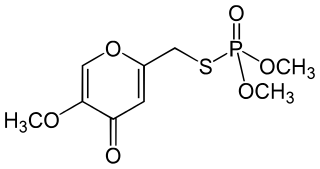
Endothion is an organic compound used as an insecticide and acaricides. It is part of the chemical class of organophosphorus compounds. It is generally described as white crystals with a slight odor. It is used as an insecticide, but not sold in the United States or Canada.
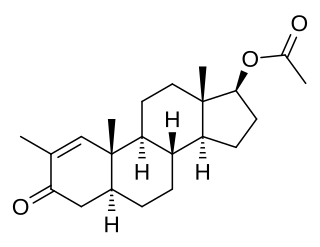
Stenbolone acetate (USAN), also known as 2-methyl-4,5α-dihydro-δ1-testosterone 17β-acetate or as 2-methyl-5α-androst-1-en-17β-ol-3-one 17β-acetate, is a synthetic, injected anabolic–androgenic steroid (AAS) and derivative of dihydrotestosterone (DHT) which has been marketed in Spain. It is the C17β acetate ester of stenbolone, which is structurally related to 1-testosterone and to drostanolone (2α-methyl-DHT).

Bolazine capronate (INN), also known as bolazine caproate or bolazine hexanoate, as well as di(drostanolone capronate) azine or 2α-methyl-5α-androstan-17β-ol-3-one 17β-hexanoate azine, is a synthetic, injected androgen/anabolic steroid (AAS) and derivative of dihydrotestosterone (DHT). It is an androgen ester – specifically, the C17β hexanoate ester of bolazine.

11β-Methyl-19-nortestosterone 17β-dodecylcarbonate (11β-MNTDC) is a synthetic and orally active anabolic–androgenic steroid (AAS) and a derivative of nandrolone (19-nortestosterone) which was developed by the Contraceptive Development Branch (CDB) of the National Institute of Child Health and Human Development (NICHD) and has not been marketed for medical use at this time. It is an androgen ester – specifically, the C17β dodecylcarbonate ester of 11β-methyl-19-nortestosterone (11β-MNT) – and acts as a prodrug of 11β-MNT in the body.

Histidine methyl ester (HME) is an irreversible histidine decarboxylase inhibitor. It is the methyl ester of histidine.
















