
In physics, cryogenics is the production and behaviour of materials at very low temperatures. A person who studies elements that have been subjected to extremely cold temperatures is called a cryogenicist.

Trinitrotoluene (; TNT), or more specifically 2-methyl-1,3,5-trinitrobenzene, is a chemical compound with the formula C6H2(NO2)3CH3. This yellow solid is sometimes used as a reagent in chemical synthesis, but it is best known as an explosive material with convenient handling properties. The explosive yield of TNT is considered to be the standard measure of bombs and the power of explosives. In chemistry, TNT is used to generate charge transfer salts.

Liquid hydrogen (LH2 or LH2) is the liquid state of the element hydrogen. Hydrogen is found naturally in the molecular H2 form.

Surface science is the study of physical and chemical phenomena that occur at the interface of two phases, including solid–liquid interfaces, solid–gas interfaces, solid–vacuum interfaces, and liquid–gas interfaces. It includes the fields of surface chemistry and surface physics. Some related practical applications are classed as surface engineering. The science encompasses concepts such as heterogeneous catalysis, semiconductor device fabrication, fuel cells, self-assembled monolayers, and adhesives. Surface science is closely related to interface and colloid science. Interfacial chemistry and physics are common subjects for both. The methods are different. In addition, interface and colloid science studies macroscopic phenomena that occur in heterogeneous systems due to peculiarities of interfaces.
Chemisorption is a kind of adsorption which involves a chemical reaction between the surface and the adsorbate. New chemical bonds are generated at the adsorbant surface. Examples include macroscopic phenomena that can be very obvious, like corrosion, and subtler effects associated with heterogeneous catalysis. The strong interaction between the adsorbate and the substrate surface creates new types of electronic bonds.
In chemistry, a hydride is the anion of hydrogen, H−, or, more commonly, it is a compound in which one or more hydrogen centres have nucleophilic, reducing, or basic properties in it. In compounds that are regarded as hydrides, the hydrogen atom is bonded to a more electropositive element or groups. Compounds containing hydrogen bonded to metals or metalloid may also be referred to as hydrides. Common examples are ammonia (NH3), methane (CH4), ethane (C2H6) (or any other hydrocarbon), and Nickel hydride (NiH), used in NiMH rechargeable batteries.

Adsorption is the adhesion of atoms, ions or molecules from a gas, liquid or dissolved solid to a surface. This process creates a film of the adsorbate on the surface of the adsorbent. This process differs from absorption, in which a fluid is dissolved by or permeates a liquid or solid, respectively. Adsorption is a surface phenomenon, while absorption involves the whole volume of the material. The term sorption encompasses both processes, while desorption is the reverse of it.
Palladium hydride is metallic palladium that contains a substantial quantity of hydrogen within its crystal lattice. Despite its name, it is not an ionic hydride but rather an alloy of palladium with metallic hydrogen that can be written PdHx. At room temperature, palladium hydrides may contain two crystalline phases, α and β. Pure α phase exists at x < 0.017 whereas pure β phase is realised for x > 0.58; intermediate x values correspond to α-β mixtures.
The hydrogen economy is the use of hydrogen as a fuel, particularly for electricity production and hydrogen vehicles; and using hydrogen for long term energy storage and for long distance transport of low-carbon energy.

Grid energy storage is a collection of methods used to store electrical energy on a large scale within an electrical power grid. Electrical energy is stored during times when production exceeds consumption, and returned to the grid when production falls below consumption.

Pressure swing adsorption (PSA) is a technology used to separate some gas species from a mixture of gases under pressure according to the species' molecular characteristics and affinity for an adsorbent material. It operates at near-ambient temperatures and differs significantly from cryogenic distillation techniques of gas separation. Specific adsorbent materials are used as a trap, preferentially adsorbing the target gas species at high pressure. The process then swings to low pressure to desorb the adsorbed material.
Hydrogen technologies are technologies that relate to the production and use of hydrogen. Hydrogen technologies are applicable for many uses.
A hydrogen sensor is a gas detector that detects the presence of hydrogen. They contain micro-fabricated point-contact hydrogen sensors and are used to locate hydrogen leaks. They are considered low-cost, compact, durable, and easy to maintain as compared to conventional gas detecting instruments.

Metal–organic frameworks (MOFs) are a class of compounds consisting of metal ions or clusters coordinated to organic ligands to form one-, two-, or three-dimensional structures. They are a subclass of coordination polymers, with the special feature that they are often porous. The organic ligands included are sometimes referred to as "struts", one example being 1,4-benzenedicarboxylic acid (BDC).

CryoSat-2 is a European Space Agency environmental research satellite which was launched in April 2010. It provides scientists with data about the polar ice caps and tracks changes in the thickness of the ice with a resolution of about 1.3 centimetres.

Compressed hydrogen (CH2, CGH2 or CGH2) is the gaseous state of the element hydrogen kept under pressure. Compressed hydrogen in hydrogen tanks at 350 bar (5,000 psi) and 700 bar (10,000 psi) is used for mobile hydrogen storage in hydrogen vehicles. It is used as a fuel gas.
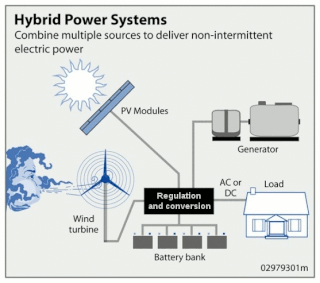
Wind hybrid power systems combines wind turbines with other storage and/or generation sources. One of the key issues with wind energy is its intermittent nature. This has led to numerous methods of storing energy.

In heterogeneous catalysis, hydrogen molecules can be adsorbed and dissociated by the metal catalyst. Hydrogen spillover is the migration of hydrogen atoms from the metal catalyst onto the nonmetal support or adsorbate. Spillover, generally, is the transport of a species adsorbed or formed on a surface onto another surface. Hydrogen spillover can be characterized by three major steps, the first being where molecular hydrogen is split via dissociative chemisorption into its constitutive atoms on a transition metal catalyst surface, followed by migration from the catalyst to the substrate, culminating in their diffusion throughout the substrate surfaces and/or in the bulk materials.












